Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Native Starch refers to unmodified purified forms of starch suitable for use as a pharmaceutical excipient. Starch is a large molecular weight polymer of α-D-glucopyranose units (general molecular formula (C6H10O5)n) widely found in plants as a reserve carbohydrate. Starch is, in effect, a mixture of two glucans: amylose and amylopectin, the composition of which depends on the plant source. In the pharmacopoeias, many different starches are recognised, including Maize (Corn) starch, Potato starch, Rice starch, Tapioca starch, Pea starch, and Wheat starch. Pharmaceutical-grade starch is available as a white, odourless and tasteless, fine powder made of small spherical or ovoid granules.
Pharmacopoeial Compliance: USP-NF; Ph.Eur; B.P; J.P; I.P
Synonyms and Trade Names: Starch; Amidon; Amylum; C*Pbarmgel®; Euryion®; Fecule®; Hylon®; Melojel®; Meritena® 141; Pearl®; Perfectamyl®; Pure-Dent®; Purity 21®; Purity® 826; Uni-Pure®
Uses and Applications: Tablet and Capsule Diluent; Tablet and Capsule Disintegrant; Tablet Binder; Thickening Agent; Dusting Powder; and Lubricant

Within the processing industries, the term ‘Native Starch’ refers to purified, unmodified grades of Starch, a complex carbohydrate derived principally from Maize (corn), Potato, Cassava, Peas, Rice or Wheat. It is distinct from modified starch, such as Pregelatinised starch, which is chemically or physically changed to impart additional functionality that is otherwise absent in Native starch.
In the USP-NF, Native Starch is listed in several individual monographs, namely Corn (Zea mays), Potato (Solatium tuberosum), Tapioca (Manihot utilissima Pohl), and Wheat starch (Triticum aestivum}. The Ph.Eur has monographs for each of these starches, except Tapioca Starch, along with additional monographs for Pea (Pisi amylum) and Rice starch (Qryza sativa). The B.P similarly describes Corn (maize), Potato, Rice, Tapioca, and Wheat starch in individual monographs. The J.P similarly describes Corn, Potato, Rice, and Wheat starch in separate monographs.
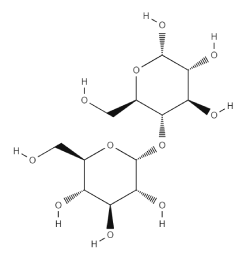
From a chemical perspective, Starch can be described as a long-chain polysaccharide polymer of α-D-glucopyranose units. It has the basic formula (C6H10O5)n, where n varies between 1000 and 10 000 (molecular weight 10-700 MDa). In reality, Starch is composed of two types of α-D-glucan chains, amylose and amylopectin, the relative content of which depends on the plant source. Amylose, which is water-soluble, is made up of helical but unbranched chains of glucose residues with α (1,4) linkages. Amylopectin, on the other hand, is water-insoluble and consists of branched glucose residues in α (1,4) and α (1,6) bonds.
Note that starch molecules produced by each plant species have specific structures and compositions (such as the length of glucose chains or the amylose/amylopectin ratio), and the protein and fat content of the storage organs may vary significantly. Generally, normal starches contain 70-80% amylopectin and 20-30% amylose. Waxy starches may have up to 99% amylopectin content.
For industrial purposes, Starch is extracted from specific plant sources following designated processes. Typical production steps are steeping (corn), wet milling (corn, potato), dry milling (wheat), or sieving and physical separation with hydrocyclones. The last production step is usually a centrifugal separation from the starch slurry followed by drying with hot air. The starch separation process may use sulphur dioxide or peroxides as a processing aid, improving the separation process and the microbial quality of the final product.
Starch is one of the most important food sources on earth, and thanks to technological developments, it also functions as a versatile material for use in the food, textile, pharmaceutical, and chemical industries. The excipient grade material is supplied as an odourless and tasteless, fine, white or off-white powder. Under close examination, it appears as small spherical granules whose shape varies depending on the botanical source of the material.
| Chemical Name | Starch |
| CAS Registry Number | [9005-25-8] |
| Empirical Formula | (C6H10O5)n.(n = 300-1 000) |
| Molecular Weight | 10-700 MDa |
| EC/ECS Number | N/A |
| UNII Code (FDA) | O8232NY3SJ |
Starch is approved as an excipient for use in tablets and capsules (swallow and buccal) as well as in powders, suspensions; topical preparations; and vaginal tablets. It is official in all the major pharmacopoeia across the world. Being part of the human and animal diet, starch is classified as a food ingredient rather than a food additive. Starch is also used in topical medical devices, nutraceuticals, and cosmetic formulations. It is GRAS listed and included in the FDA Inactive Ingredients Database.
| Form | Solid (powder) |
| Appearance | fine, white to off-white powder |
| pH value | Aqueous dispersions of Native Starch have a pH in the range 4.0-8.0 |
| Amylose content | 24-28% (Corn Starch)
35-39% (Pea Starch) 20-23% (Potato Starch) 17-20% (Tapioca Starch) 24-28% (Wheat Starch) |
| Compactability | Native Starches exhibits plastic deformation (rather than brittle fracture) when subjected to compressive stress.
The tensile strength of compacts follows the following pattern: Potato Starch > Corn Starch > Wheat Starch |
| Bulk density | Varies by production process and humidity conditions. Generally:
0.45-0.58g/cm3 (Corn Starch) 0.56-0.82 g/cm3 (Potato Starch) ≈0.50 g/cm3 (Wheat Starch) |
| Tapped density | Varies by production process and humidity conditions. Generally:
0.69-0.77 g/ml ( Corn Starch) 0.80-0.90 g/ml (Potato Starch) ≈0.76 g/ml (Wheat Starch) |
| Density | (True) 1.478 g/ml (Corn Starch) |
| Flowability | Commercial Native Starch is generally cohesive and has poor flow characteristics. The flow properties depend strictly on the moisture content. Drying can result in a free-flowing material |
| Gelatinization temperature (measured at 20% w/w in water with differential scanning colorimetry (peak) | 71 oC (Corn Starch)
62 oC (Pea starch) 64 oC (Potato Starch) 68 oC (Rice Starch) 59 oC (Wheat Starch) Gelatinization causes the rupture of the starch grains and is an irreversible loss of the structure of the starch particle |
| Moisture Content | All grades of Native Starch are hygroscopic and absorb atmospheric moisture to reach the equilibrium humidity. The approximate equilibrium moisture is characteristic for each starch. At 50% relative humidity is generally as follows:
12% (Corn Starch) 14% (Pea Starch) 18% (Potato Starch) 14% (Rice Starch); 13% (Wheat starch) Low moisture Native Starches having a humidity lower than the equilibrium humidity are commercially available. |
| Particle Size distribution | Corn Starch: 2-32 um; average particle diameter 13 um
Pea Starch: 5-90 um; average particle diameter 30 um Potato Starch: 10-100 um; average particle diameter 46 um Rice Starch: 2-20 um; average particle diameter 5 um Tapioca Starch: 5-35 um; average particle diameter 13 um Wheat Starch: 2-45 um. It exhibits a bimodal particle size distribution, peak values approx. 2um and 20 m |
| Solubility | Native Starch is practically insoluble in cold ethanol and cold water. Starch swells instantaneously in water by about 5-10% at 37 oC. Starch is soluble in hot water at temperatures above the gelatinization temperature |
| Specific Surface Area | 0.40-0.54 m2/g (Corn Starch) |
| Swelling temperature | Swelling is a reversible process
64 oC (Corn Starch) 63 oC (Potato Starch) 72 oC (Rice Starch) 55 oC (Wheat Starch) |
| Viscosity | Aqueous pastes of Native Starches exhibit non-Newtonian viscosity behaviour. Viscosity can be ranked as follows: Potato Starch > Tapioca Starch > Corn Starch. At concentrations above 40%, Native Starch dispersions exhibit significant rheopexy |
| USP-NF | PhEur | JP | |
| Name | specified | specified | specified |
| Authorised use | Excipient | Excipient | Excipient |
| Definition | specified | specified | specified |
| Identification | specified | specified | specified |
| Characters | specified | specified | n/a |
| pH | |||
| Corn starch | 4.0 -7.0 | 4.0 -7.0 | 4.0 -7.0 |
| Pea starch | n/a | 5.0 -8.0 | n/a |
| Potato starch | n/a | 5.0 -8.0 | n/a |
| Rice starch | n/a | 5.0 -8.0 | n/a |
| Tapioca starch | 4.5-7.0 | n/a | n/a |
| Wheat starch | 4.5-7.0 | 4.5 -7.0 | 4.5 -7.0 |
| Loss on drying | |||
| Corn starch | ≤ 15.0% | ≤ 15.0% | ≤ 15.0% |
| Pea starch | n/a | ≤ 16.0% | n/a |
| Potato starch | ≤ 20.0% | ≤ 20.0% | ≤ 20.0% |
| Rice starch | n/a | ≤ 15.0% | ≤ 15.0% |
| Tapioca starch | ≤ 16.0% | n/a | n/a |
| Wheat starch | ≤ 15.0% | ≤ 15.0% | ≤ 15.0% |
| Residue on ignition | |||
| Corn starch | ≤ 0.6% | n/a | ≤ 0.6% |
| Pea starch | ≤ 0.6% | n/a | ≤ 0.6% |
| Potato starch | n/a | n/a | ≤ 0.1% |
| Rice starch | ≤ 0.6% | n/a | n/a |
| Tapioca starch | ≤ 0.6% | n/a | ≤ 0.6% |
| Sulfated ash | |||
| Corn starch | n/a | ≤ 0.6% | n/a |
| Pea starch | n/a | ≤ 0.6% | n/a |
| Potato starch | n/a | ≤ 0.6% | n/a |
| Rice starch | n/a | ≤ 0.6% | n/a |
| Tapioca starch | n/a | ≤ 0.6% | n/a |
| Iron | |||
| Corn starch | ≤ 10 µg/g | ≤ 10 ppm | ≤ 10 ppm |
| Pea starch | n/a | ≤ 50 ppm | n/a |
| Potato starch | ≤ 10 µg/g | ≤ 10 ppm | ≤ 10 ppm |
| Rice starch | n/a | ≤ 10 ppm | n/a |
| Tapioca starch | ≤ 0.002% | n/a | n/a |
| Wheat starch | ≤ 10 µg/g | ≤ 10 ppm | ≤ 10 ppm |
| Oxidizing substances | |||
| Corn starch | ≤ 20 µg/g | ≤ 20 ppm | ≤ 20 ppm |
| Pea starch | n/a | ≤ 20 ppm | n/a |
| Potato starch | ≤ 20 µg/g | ≤ 20 ppm | ≤ 20 ppm |
| Rice starch | n/a | ≤ 0.002% | n/a |
| Tapioca starch | ≤ 0.002% | n/a | n/a |
| Wheat starch | 20 µg/g | 20 ppm | ≤ 20 ppm |
| Sulfur dioxide | |||
| Corn starch | ≤ 50 µg/g | ≤ 50 ppm | ≤ 50 ppm |
| Pea starch | n/a | ≤ 50 ppm | n/a |
| Potato starch | ≤ 50 µg/g | ≤ 50 ppm | |
| Rice starch | n/a | ≤ 50 ppm | n/a |
| Tapioca starch | ≤ 0.005% | n/a | n/a |
| Wheat starch | ≤ 50 µg/g | ≤ 50 ppm | ≤ 50 ppm |
| Total protein | |||
| Wheat starch | ≤ 0.3% | ≤ 0.3% | n/a |
| Foreign matter | |||
| Corn starch | n/a | specified | n/a |
| Pea starch | n/a | specified | n/a |
| Potato starch | n/a | Specified | n/a |
| Rice starch | n/a | Specified | specified |
| Wheat starch | n/a | specified | n/a |
| Microbial limits | specified | specified | n/a |
| Assay | n/a | n/a | n/a |
| Labelling | specified | n/a | n/a |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Native starches are versatile excipients that are widely used in oral solid-dosage formulations (where they function as binders, diluents, and disintegrating agents) and topical drug, medical devices and cosmetic products.
When selected for use as diluents, Starches are utilised to prepare standardized triturates of pigments and colourants, highly potent drug substances, and herbal extracts, thereby allowing efficient blending during product manufacturing.
Starches are also utilised in hard capsule formulations to adjust fill volume. Owing to its inherent lubricity, Starch quantities of 3-10% w/w can act as an antiadherent and lubricant in tableting and capsule filling.
When used as a wet granulation binder in tablet formulations, a freshly prepared starch paste can be added at a concentration of 3-20% w/w (usually 5-10%, depending on the starch type). It is recommended that the amount required (the binder ratio) be established through optimization studies, utilising tablet parameters including friability and hardness, disintegration time, and the rate of dissolution.
As a disintegrating agent, Starch remains one of the most commonly used excipients. Typical concentrations 3-25% w/w; although the ideal concentration is 15%. When using Starch, it is important to have a prior granulation step to avoid problems with insufficient flow and segregation.
However, Native starch does not compress well and has a tendency to increase tablet friability and capping especially when used in high concentrations. It has been shown that balancing the elastic properties of starch with specific excipients can significantly improve the compaction properties during tableting.
Rice and Wheat starch are also used in a number of topical formulations taking advantage of their absorbency of liquids. Starch paste can also be used in ointment formulations, normally in combination with Glycerin.
Starch has also been widely investigated as an excipient in novel drug delivery systems for colon, nasal, and other site-specific delivery systems. Starches are useful carriers for amorphous drug preparations, as well for the formulation of multiparticulates (pellets) for immediate or delayed drug release applications. It can also improve the bioavailability of poorly soluble actives.
Starch, particularly Rice starch, has also been used in the treatment of children’s diarrhoeal diseases, particularly in resource-poor settings. Specific Starch varieties with a high amylose content (resistant starches) are used as insoluble fibre in clinical nutrition, and also for colon-targeting applications.
Native Starches that comply with stipulated pharmacopoeia specifications serve as important raw materials for the production of many other Starch-based excipients and active pharmaceutical ingredients, that are separately covered under their own pharmacopoeia monographs.
Starch, being an edible food substance, is regarded as an essentially nontoxic and non-irritant material. Both amylose and amylopectin have been evaluated as safe and without limitation for daily intake. Allergic reactions to starch are extremely rare and individuals apparently allergic to one particular starch may not experience adverse effects with starch from a different botanical source. Wheat proteins (gluten) in starch, however, are problematic for conditions such as celiac disease. In this case, Tapioca starch may be a viable option.
Starch has been used as glove powder since the 1950s. However, contamination of surgical wounds with the starch glove powder used by surgeons has resulted in the development of granulomatous lesions.
Toxicology: LD50 (mouse, IP): 6.6g/kg
Starch is a physically stable material and provided it is protected from high humidity, will remain viable for a long time period. Starch is considered to be chemically and microbiologically inert under normal storage conditions. The standard shelf life is given as 24-36 months. However, Starch solutions or pastes are physically unstable and are readily metabolized by microorganisms. They should therefore be freshly prepared when used for wet granulation. Starch should be stored in an airtight container in a cool, dry place.
When handling Starch, you should observe normal precautions appropriate to the circumstances and quantity of material handled. Eye protection and a dust mask are recommended. Excessive dust generation should be avoided to minimize the risks of explosion. The minimal explosive concentration of corn starch is 30-60 g/m³ air. The long-term (8-hour TWA) workplace exposure limits for starch are 10 mg/m3 for total inhalable dust and 4 mg/m3 for respirable dust.
Native Starch is sourced from sustainable plant sources. As a biodegradable, natural substance, it is considered safe for the environment with no long-term impact on ecology or marine life. Starch excipient grade achieved a total score of 84/100 by the Excipients Forum Sustainable Chemistry Score.™
Ingredion Corporation
[4] J.C. Callahan, G.W. Cleary, M. Elefant, G. Kaplan, T. Kensler, R.A. Nash, Equilibrium moisture content of pharmaceutical excipients, Drug Development and Industrial Pharmacy, 8 (1982) 355-369.
[5] The U.S.P. Convention, Food Chemicals Codex (11th Edition), (2018).
[7] T. Durig, Binders in pharmaceutical granulation, Handbook of Pharmaceutical Granulation Technology, (2009) 78-97.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.