Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Pregelatinised Starch is a type of Starch excipient obtained through the hydrothermal modification of Native starch which raptures all or some of the starch granules. The source of starch may be Maize (corn), Potato, Wheat starches or blends. Compared with Native starch, Pregelatinised Starch has improved functionality, including flowability, compressibility, and cold water solubility. It is available as a white, odourless and tasteless, moderately fine powder made of small spheric or ovoid granules.
Pharmacopoeial Compliance: USP-NF; Ph.Eur; B.P; J.P; I.P
Synonyms and Trade Names: Pregelatinised Starch; Compressible Starch; C*PharmGel®; Insta-starch®; Lycatab® C; Lycatab® PCS; Merigel®; National® 78-1551; Pharma-Gel®; Prejel®; Sepistab® ST200; Starch® 1500 G; Unipure® WG220.
Uses and Applications: Tablet and Capsule Diluent; Tablet and Capsule Binder; Disintegrating Agent; Dusting Powder; and Lubricant
Starch is the principal carbohydrate in all higher plants. The most important sources today are cereals (corn and wheat), tubers (potato and potato), and legumes (pea). In the Native (non-pregelatinised) form, starch is insoluble in water and consists of granules whose sizes, type and physicochemical properties vary depending on the botanical source.
Pregelatinised starch is defined as a modified Starch grade. It is obtained by gelatinising and drying an aqueous slurry (42% w/w) of Native starch at 62-72 oC followed by drying and grading. Gelatinization is a physical process in which starch granules are raptured, a process that improves the product’s properties such as cold water solubility, viscosity, and moisture uptake. Currently, Maize (corn) is the most commonly used starch in the production of Pregelatinized starch.
Note that both fully and partially pregelatinized grades are commercially available. Partial pregelatinization renders the starch flowable and directly compressible whereas full pregelatinization produces a cold-water-soluble starch that can be used as a wet granulation binder. Typically, pregelatinized starch contains 5% of free amylose, 15% of free amylopectin, and 80% unmodified starch.
The USP-NF does not specify the botanical origin of the original starch. The Ph.Eur, however, does specify that pregelatinized starch is obtained from maize (corn], potato, or rice starch. Normally the fully pregelatinized starch contains 20-30% amylose and the rest amylopectin, which is about the same ratio (1: 3) as for the partially pregelatinized form.
Food-grade Pregelatinised starches are slightly different. They are prepared by heating an aqueous slurry containing up to 42% w/w of starch at 62-72 oC. Chemical additives that may be included in the slurry are gelatinization aids (salts or bases) and surfactants, added to control rehydration or minimize stickiness during drying. After heating, the slurry may be spray-dried, roll-dried, extruded, or drum-dried. In the last case, the dried material may be processed to produce a desired particle size range.
Pharmaceutical fully pregelatinized starches use no additives and are prepared by spraying an aqueous suspension of ungelatinized starch on hot drums where gelatinization and subsequent drying take place. Partially pregelatinized starch is produced by subjecting moistened starch to mechanical pressure. The resultant material is ground and the moisture content is adjusted.
Pregelatinized starch occurs as a moderately coarse to fine, white to off-white coloured powder. It is odourless and has a slight characteristic taste. The pharmacopoeia describes pregelatinised starch as starch, other than wheat starch, that has been mechanically processed in the presence of water, with or without heat to rapture all or part of the starch granules. It contains no added substances but may be modified to improve flow or compressibility. When examined under a polarising microscope, pregelatinized starch as a slurry in cold water, reveals no significant ungelatinized granules, i.e. no ‘maltese crosses’ characteristic of the starch birefringence pattern. Partially pregelatinized starch (e.g. Starch 1500G and Sepistab ST200) show retention of birefringence patterns typical of unmodified starch granules.
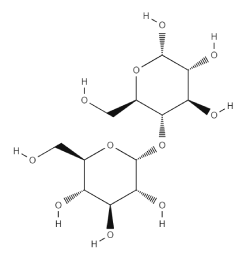
| Chemical Name | Starch |
| CAS Registry Number | [9005-25-8] |
| Empirical Formula | (C6H10O5)n.(n = 300-1 000) |
| Molecular Weight | 10-700 MDa |
| EC/ECS Number | N/A |
| UNII Code (FDA) | O8232NY3SJ |
Pregelatinised Starch is an approved pharmaceutical excipient. It is listed in the USP-NF, Ph.Eur, B.P; J.P, and I.P. It is also included in the FDA Inactive Ingredients Database (oral capsules, suspensions, and tablets; vaginal preparations). Pregelatinized starch and native starch are widely used in oral solid-dosage formulations.
| Form | Solid, powder |
| Appearance | White or yellowish-white powder |
| Density (tapped) | 0.879 g/cm3 |
| pH value | n/a |
| pKa | n/a |
| Log P | n/a |
| Density (true) | 1.516g/cm3 |
| Flowability | 18-23% (Carr compressibility index) |
| Moisture content | Pregelatinized maize starch is hygroscopic |
| Particle size distribution | 30-150 µm, median diameter 52 µm. For partially pregelatinized starch, greater than 90% through a US #100 mesh (149 μm); and less than 0.5 % retained on a US #40 mesh (420 μm). |
| Solubility | Slightly soluble to soluble in cold water, depending upon the degree of pregelatinization. Pastes can be prepared by sifting the pregelatinized starch into stirred, cold water. Practically insoluble in organic solvents |
| Specific surface area | 0.26 m2 /g (Starch 1500® Colorcon); 0.18-0.28 m2 /g (Roquette) |
| Viscosity (dynamic) | 8-10mPa s (2% w/v aqueous dispersion at 25 °C) |
| Test | Specification | Reference |
| Name | Pregelatinised starch | PhEur/USP-NF |
| Authorised use | Excipient | PhEur/USP-NF |
| Characters | White or yellowish-white powder | PhEur/USP-NF |
| Identification | A, B | PhEur/USP-NF |
| pH | 4.5 – 7.0 | PhEur/USP-NF |
| Iron | ≤20 ppm | PhEur/USP-NF |
| Oxidising substances | specified | PhEur/USP-NF |
| Foreign matter | specified | PhEur/USP-NF |
| Loss on drying | ≤15.0% | PhEur/USP-NF |
| Sulphated ash | ≤6.0% | PhEur/USP-NF |
| Microbial contamination | specified | PhEur/USP-NF |
| Assay | n/a | Ph.Eur/USP-NF |
| Labelling | specified | Ph.Eur/USP-NF |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Pregelatinised starch is a multifunctional excipient that can be used as a tablet and capsule filler/diluent, wet granulation binder, and disintegrating agent. It is mainly used in the formulation of solid dosage forms (tablets and capsules).
Modification of native starch through pregelatinisation improves functionality in three key respects: flowability; solubility and binding effects, and disintegrating capabilities. Pregelatinised starch can be further modified through spray-drying or co-processing with Microcrystalline cellulose to further improve compressibility (Co-processed Starch and MCC, and Co-processed Starch and Lactose).
Thanks to its enhanced flowability and compression characteristics over native starch, it can be used as a filler and dry binder dry-compression or direct compression processes. It is recommended that levels of Pregelatinised starch be no more than 25% (ideally between 15 and 17%). Using levels above this range can impact tablet strength even when high compression pressures are used.
Pregelatinized starch is also self-lubricating. However, when it is used with other excipients it is often necessary to add a lubricant to the formulation. Although magnesium stearate (at 0.25% w/w) is commonly used for this purpose, concentrations greater than this may have adverse effects on tablet strength and dissolution. Therefore, stearic acid is generally the preferred lubricant with pregelatinized starch. Partially pregelatinized starch is used in oral dry powder hard capsule formulations. Both partially and fully pregelatinized starch may also be used in wet granulation processes.
Fully Pregelatinised starch is mainly used as a binder in wet granulation processes taking advantage of the higher content of soluble fractions and tackiness. When starch is full pregelatinised, it loses its disintegrant properties, which limits its application scope.
Various studies have reported nuances in the different grades of Pregelatinised starch. For instance, Starch 1500® (Colorcon) exhibits marginally better flowability compared with Lycatab C (Roquette) but conversely, Lycatab C has higher plasticity and compacts better, which results in stronger tablets. However, it is also more sensitive to magnesium stearate levels than Starch 1500. You can read more about these differences in Svačinová P et al, Starch 2021, 73, 2000166 (https://doi.org/10.1002/star.202000166).
Pregelatinized starch is derived from native starch, a material generally regarded as nontoxic and non-irritant. However, oral consumption of large amounts of pregelatinized starch may be harmful. See Starch for further information.
Pregelatinized starch is a stable but hygroscopic material. The assigned shelf-life is 36 months. It is hygroscopic and should be stored in a well-closed container in a cool, dry place.
When handling Pregelatinised starch, you should observe normal precautions appropriate to the circumstances and quantity of material handled. Eye protection and a dust mask are recommended. Excessive dust generation should be avoided to minimize the risks of explosions.
In the UK, the long-term (8-hour TWA) workplace exposure limits for starch are 10mg/m3 for total inhalable dust and 4mg/m3 for respirable dust.
A Sustainability score for Pregelatinised Starch has not been provided.
Colorcon Inc.
Ingredion (ex National Starch)
[5] The U.S.P. Convention, Food Chemicals Codex (11th Edition), (2018).
[6] A. Crouter, L. Briens, The Effect of Moisture on the Flowability of Pharmaceutical Excipients, AAPS PharmScitech, 15 (2014) 65-74.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.