Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Methacrylic Acid-Ethyl Acrylate Copolymer (1:1) is a synthetic anionic copolymer of methacrylic acid and ethyl acrylate (molecular weight 320,000 g/mol) for use as an enteric coating (trigger pH 5.5). It is commonly known as EUDRAGIT® L100-55 (Evonik) or KOLLICOAT MAE 100P (BASF). Methacrylic acid-Ethyl acrylate copolymer (1:1) is obtained by free-radical polymerisation of acrylate and methacrylate derivatives and is supplied as a free-flowing powder with a faint acrylic odour.
Synonyms and Trade Names: Methacrylic Acid–Ethyl Acrylate Copolymer (1:1) is also known as Poly(methacrylic Acid Ethyl Acrylate) 1:1; Acrylates Copolymer; Methacrylic Acid Copolymer, Type C; Methacrylic Acid Copolymer LD; EUDRAGIT® L100-55; Kollicoat® MAE 100 P; Acryl-EZE; ACRYCOAT L100-55
Pharmacopoeial Compliance: USP-NF; Ph.Eur; JP; IP
Uses and Applications: Enteric Polymer; Film Forming Agent; Tablet Binder; and Tablet Coating Agent
Methacrylic Acid–Ethyl Acrylate Copolymer (1:1) is a synthetic anionic polymethacrylate copolymer derived from esters of acrylic and methacrylic acid. It contains between 46% and 56% m/m methacrylic acid units, and has a mean relative molecular mass of approximately 320 000, and the ratio of carboxylic groups to ester groups is 1:1. The presence of carboxylic acid groups in its structure renders Methacrylic Acid–Ethyl Acrylate Copolymer (1:1) insoluble in acidic media but soluble in neutral to alkaline fluids, making it ideal for use as an enteric film coating agent. It is supplied as a solid substance in form of a white, free-flowing powder with a faint characteristic odour.
Poly(methacrylate) copolymers have a long history of use in the pharmaceutical industry. They were first introduced in 1955 following on from the work of the famed scientist, Otto Rohm (Rohm & Haas), who also invented PLEXIGLASS®, a material that is chemically related to and shares many chemical properties with poly(methacrylate) copolymers. Their introduction marked a step-change in the field of drug delivery and pharmaceutical technology owing to their reproducibility and versatility compared with naturally derived polymers based on cellulose.
Basics of Poly(methacrylate) Copolymers
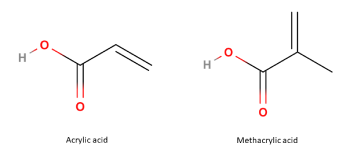
The structural basis of all Poly(methacrylate) copolymers, such as Methacrylic Acid–Ethyl Acrylate Copolymer, is Methacrylic acid, an alpha, beta-unsaturated monocarboxylic acid, that is, acrylic acid having the hydrogen at C2 substituted by a methyl group:
To synthesise poly(methacrylate) polymers, acrylic and methacrylic acid esters are combined via free radical polymerisation. Long polymer chains are then formed by chain grow reactions from various acrylate or methacrylate derivatives. The polymerisation reaction can be performed in a solvent, bulk, suspension or emulsion:
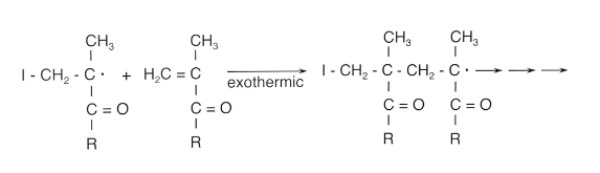
Variations in chain lengths can be obtained via termination and transfer reactions, while the functional properties of methacrylic copolymers and the final polymers are adjusted by selecting from a variety of monomers. Generally, non-functional comonomers determine the polymer’s properties while functional comonomers determine the solution profile.
Classification of Poly(methacrylate) Copolymers
Depending on the functional group attached to the polymer chain backbone, poly(methacrylate) copolymers can be broadly categorised into two groups:
In reality, significant overlap exists and the ideal method for classifying poly(methacrylate) copolymers is to use both schemes as shown below:

Anionic Polymers
Anionic poly(methacrylate) polymers contain -COOH (methacrylic acid) functional groups, which allows them to dissociate and dissolve at the higher pH of the small intestine and colon. These polymers offer enteric protection to many active ingredients in oral solid formulations in the gastric environment and can trigger drug release at a selected pH for targeted drug delivery. They are available in a wide range of physical forms (aqueous dispersions, organic solvent, granules, and fine powders).
Cationic Polymers
Cationic polymethacrylate polymers are based on dimethyl aminoethyl methacrylate, butyl methacrylate, and methyl methacrylate residues. These grades are cationic and soluble in gastric fluid below pH 5 but become swellable and permeable, but not soluble, above pH 5. For this reason, cationic poly(methacrylate) copolymers can be used for taste-masking and moisture-protection applications. They are available in a wide range of physical forms, including aqueous dispersions, organic solvents, granules, and fine powders.
Neutral Polymers
Neutral poly(methacrylate) copolymers are further divided into two broad groups:
A summary of the various poly(methacrylate) copolymer grades, their names and their application scope in pharmaceuticals is shown in the table below:


R = COOH
Alkyl = -CH2CH3
| Chemical Name | Poly(methacrylic acid co-methyl methacrylate) 1:1 |
| CAS Registration Number | [25212-88-8] |
| Empirical Formula | n/a |
| Molecular weight | 250 000 |
| EC Number | n/a |
| UNII Code (FDA) | T967IEU43C |
Methacrylic Acid–Ethyl Acrylate Copolymer (1:1) is an approved pharmaceutical excipient. It is listed in the Ph.Eur (Methacrylic Acid–Ethyl acrylate copolymer (1:1)), USP-NF (Methacrylic Acid Copoymer, Type C) and the J.P.E (Methacylic Acid Copolymer LD). It is included in the US FDA Inactive Ingredients Database. It is also approved for food use in the EU (E1207).
| Physical state | Solid |
| Appearance | White fine powder |
| Acid value (mg KOH/g DS) | 300-330 |
| pKa | No data |
| Log P | No data |
| Poured density | 0.350-0.42 g/cm3 |
| Tapped density | 0.418-0.452 g/cm3 |
| Density (true) | 0.820-0.850 g/cm3 depending on grade/supplier |
| Solubility | Insoluble in water and acids. Soluble in Acetone, Ethanol, Ethyl acetate, Sodium hydroxide solutions & intestinal fluids above pH 5.5 |
| Viscosity (dynamic) | 10-200 cP |
| Test | USP-NF | Ph.Eur | B.P |
| Name | Methacrylic acid and ethyl acrylate copolymer | Methacrylic acid-ethyl acrylate copolymer 1:1 | Methacrylic acid-ethyl acrylate copolymer 1:1 |
| Authorised use | Excipient | Excipient | Excipient |
| Definition | specified | specified | specified |
| Characters | White, free-flowing powder | White, free-flowing powder | White, free-flowing powder |
| Identification | A
B |
A
B |
A
B |
| Appearance of a film | n/a | specified | specified |
| Viscosity | 100-200 mPa.s | 100-200 mPa.s | 100-200 mPa.s |
| Ethyl acrylate and methacrylic acid | n/a | ≤0.1% | ≤0.1% |
| Heavy Metals | ≤0.002% | n/a | n/a |
| Loss on Drying | ≤4.0% | ≤5.0% | ≤5.0% |
| Sulphated Ash | n/a | ≤0.4% | ≤0.4% |
| Assay | 46-50.6% (methacrylic acid units) | 46-50.6% (methacrylic acid units) | 46-50.6% (methacrylic acid units) |
| Labelling | specified | n/a | n/a |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Methacrylic Acid–Ethyl Acrylate Copolymer (1:1) is an enteric film forming agent and tablet binder. It is used in oral capsule and tablet formulations as film-coating agents. Methacrylic Acid–Ethyl Acrylate copolymer (1:1) may additionally be used to form the matrix tablet systems. The benefits of using Methacrylic Acid –Ethyl acrylate copolymer (1:1) are:
Note that for enteric applications, there are several anionic Poly(methacrylate) copolymers that possess carboxyl groups in varying amounts, and therefore, suitable for use, either alone or in combination with Methacrylic Acid-Ethyl Acrylate copolymer (1:1), as enteric coatings. When the dissolution pH is reached, the polymers dissolve by salt formation, allowing the drug substance to be released in the GI tract.
| Pharmacopoeial name | Brand | Solubility | Release site |
| Methacrylic acid-ethyl acrylate copolymer (1:1) | EUDRAGIT®L30 D-55
EUDRAGIT®L 100-55 |
≥pH 5.5 | Duodenum |
| Methacrylic acid-methyl acrylate copolymer (1:1) | EUDRAGIT® L 100
EUDRAGIT® L 12.5 |
≥pH 6.0 | Jejunum |
| Methacrylic acid-methyl acrylate copolymer (1:2) | EUDRAGIT® S 100
EUDRAGIT® S 12.5 |
≥pH 7.0 | Ileum/colon |
| Poly(methyl acrylate-co-methyl methacylate-co-methacrylic acid) 7:3:1 | EUDRAGIT® FS30D | ≥Ph 7.0 | Ileum/colon |
If the interest is in simple enteric coatings that dissolve quickly in the small intestine, the aqueous dispersion, Methacrylic acid-ethyl acrylate copolymer (1:1) 30% dispersion or or the same polymer in spray-dried form, Methacrylic acid-ethyl acrylate copolymer (1:1) are more than adequate. If the drug is to be released lower down in the small intestines, poly(methacrylate) copolymers with higher trigger pH, such as Methacrylic acid-methyl acrylate copolymer (1:1) or Methacrylic acid-methyl acrylate copolymer (1:2) can be used in different mixtures to create specific dissolution profiles.
For release in the colon, grades with dissolution pH of 7 are used. Methacrylic acid-methyl acrylate copolymer (1:2) (e.g EUDRAGIT® S), is the choice for coating tablets while the Poly(methyl acrylate-co-methyl methacrylate-co-methacrylic acid) 7:3:1 (e.g EUDRAGIT® FS 30D) is recommended for coating multiparticulates due to its greater flexibility.
Preparation of a coating dispersion in water by redispersion of Methacrylic acid-ethyl acrylate copolymer (1:1)
Methacrylic Acid-Ethyl Acrylate copolymer (1:1) is the dry substance of Methacrylic Acid-Ethyl Acrylate copolymer (1:1) 30% dispersion in a re-dispersible form. The redispersion is achieved by the addition of small amount of alkali or organic bases to produce an aqueous suspension of the powder. In this process, about 6 mol-% of the carboxyl groups in the polymer are neutralised; the resistance to gastric juice of the resulting polymer remains unimpaired. The resulting dispersion exhibits similar properties and can be used much the same way as Methacrylic acid-ethyl acrylate copolymer (1:1) 30% dispersion.
Dispersing procedure
A simple stirrer is more than adequate for this task, which can be accomplished at moderate speed. The powder should be added into the water and stirred in a sufficiently moderate speed to prevent sedimentation, being careful not to introduce air.
The following formulation is for 1 kg (sufficient for 10 kg)
| Eudragit L 100-55 | 300 g | 30.0 % |
| 1 N Sodium hydroxide solution | 100 g | 0.4 % |
| Water | 600 g | 69.6% |
| 1000 g | 100.0 % |
The sodium hydroxide solution can be prepared by dissolving 4g of sodium hydroxide pellets in water. 600 g of water is poured into a 2-litre vessel, and 300 g of Eudragit L 100-55 is stirred in portions, ensuring that the powder is thoroughly wetted and no lumps are formed. After stirring for 5–10 mins, the sodium hydroxide is added dropwise and stirred for 5 mins.
Stirring is continued for approx. 30 mins. After this time, a latex-like dispersion is formed, recognised by the formation of a milk-like consistency. This should be filtered by passing it through a 0.25 mm sieve. In the event of foam formation, 2-3 drops of Simethicone-based antifoaming emulsion can be added.
Supplementary ingredients, such as plasticizers, pigments (Titanium dioxide) and anti-tacking agents (Talc) can then be added in much the same way as Methacrylic Acid-Ethyl Acrylate Copolymer (1:1) 30% Dispersion (e.g Eudragit L30 D-55).
Methacrylic Acid–Ethyl Acrylate Copolymer (1:1) is a long-established synthetic polymer that is widely used as an enteric film-coating material in oral pharmaceutical formulations. It is not absorbed or metabolised by the body following oral ingestion. For this reason, it is generally regarded as non-toxic and non-irritant material when used as intended in the specified dosage ranges. A daily intake of 2-200 mg/kg body weight (depending on polymer type) has been set by authorities based on data from chronic oral toxicity studies in animal models.
Toxicology: LD50 (rat, SC): 75g/kg; Carcinogenicity – Not shown.
Methacrylic acid–ethyl acrylate copolymer (1:1) is stable at temperatures <30 °C. Shelf life is assigned as 24-36 months when stored in accordance with the manufacturer’s recommendations. Storage at elevated temperature tends to make powders clump, although this does not affect the quality of the material and any formed clumps are easily disaggregated.
Care should be taken when handling Methacrylic acid copolymers in the workplace. Acute and chronic adverse effects have been reported in individuals who routinely handle closely related substances (Methyl methacrylate and poly(methyl methacrylate). In several countries, the workplace exposure limit for Methyl methacrylate has been set at 208 mg/m3 (50ppm) long-term (8-hour TWA), and 416mg/m3 (100ppm) short-term.
Observe applicable SHEQ protocols when processing Methacrylic acid-ethyl acrylate copolymer. PPE (eye protection, gloves, and a dust mask or respirator) is recommended. Adequate ventilation and procedures to reduce dust formation should be should be in place for the safety of everyone else.
Click here to read more about recommended storage conditions for EUDRAGIT L100-D and EUDRAGIT L30 D-55 aqueous dispersions here:
EUDRAGIT® L100-55 Storage Stability Information Sheet (Evonik)
A sustainability score for Methacrylic Acid–Ethyl acrylate copolymer (1:1) has not been provided.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.