Thickening agents are usually high molecular weight polymer excipients commonly used to increase viscosity of formulations. This can serve several objectives, including stability, ease of use and drug delivery. In this post, we provide an overview of materials frequently used to thicken pharmaceutical products.
Definition of Pharmaceutical Thickeners
Pharmaceutical thickening agents (thickeners, for short) are a diverse group of excipient materials used to increase the viscosity of liquid pharmaceutical systems, ideally without altering other fundamental properties.
They are also commonly known as viscosity-increasing agents.
Viscosity is the internal friction that occurs between the molecules within a liquid. It is a measure of the resistance to deformation in shear, such as flow or pouring, and is due to intermolecular friction and molecular adhesion-cohesion within the fluid.
Viscosity of a fluid decreases with increase in temperature due to increased atomic/molecular mobility, which decreases intermolecular cohesion and friction. The presence in solution of a high concentration of solutes can have an appreciable impact on viscosity of the solution.
This is why the terms “thick” (concentrated and high viscosity) and thin (dilute and low viscosity) are used as qualitative descriptions of the viscosity of liquid systems.
Many thickeners form weakly cohesive colloidal structures, which can exist as solutions, suspensions or gels when dissolved or dispersed in a suitable liquid medium.
Note that thickening agents are sometimes (and mistakenly) referred to as suspending agents. Although there is overlap in the type of excipients used for both product categories, there are sufficient differences to warrant a clear separation of the two categories, an approach we have taken in this article.
How Thickeners Increase Solution Viscosity
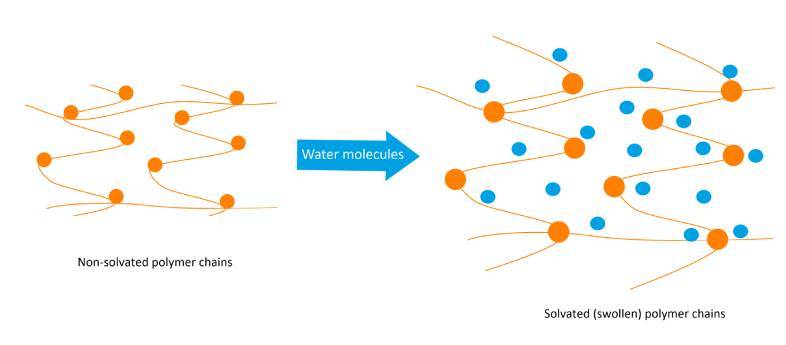
With respect to the use of polymers, the addition of a high molecular weight polymer increases viscosity of the liquid in which it is dissolved. The increase in viscosity is caused by strong internal friction between the randomly coiled and/or swollen macromolecules, and the surrounding solvent molecules, through hydrogen bonding¹. This is illustrated below:
How Thickeners Increase Solution Viscosity
With respect to the use of polymers, the addition of a high molecular weight polymer increases viscosity of the liquid in which it is dissolved. The increase in viscosity is caused by strong internal friction between the randomly coiled and/or swollen macromolecules, and the surrounding solvent molecules, through hydrogen bonding¹. This is illustrated below:
Functions of Pharmaceutical Thickeners
Viscosity is one of the most important properties associated with liquid systems, and formulations that possess high viscosity have many practical applications within the pharmaceutical space, as outlined below:
Drug delivery
The rate of drug delivery is influenced by viscosity of the milieu. Liquid and semisolid topical and oral formulations, as well as parenteral and matrix-based solid dosage forms frequently utilise high viscosity systems to slow down rates of drug diffusion.
Demulcent properties (soothing, irritation reduction & increase in slip)
Demulcents are a class of materials used to lubricate and protect mucous membranes, and more especially the oral, pharyngeal, oesophageal, and gastric mucosa. Their action depends on having sufficient tack and slip, both of which are obtained when polymeric excipients are dissolved in aqueous media. Click here to read about the challenges of dysphagia and oral medicines, and what action is required if we are to address it.
Substantivity and film formation
The ability of a formulation to adhere and remain in place (contact time) is a function of, among other factors, viscosity and film formation. Viscosity-increasing agents are routinely used to increase substantivity and contact time of topical and bioadhesive formulations.
Suspending and stability enhancement
Thickeners are typically used to minimise separation and settling that typically follow when insoluble ingredients are suspended in liquids
Improvement of appearance, consistency and quality
Thickening agents are added to skincare formulations to give skincare products a more appealing consistency and smoothness. Consistency is a particular formulation characteristic that describes the firmness or runniness of a product.
Gelling and texturizing
A key property of hydrocolloids (such as pectin and xanthan gum) is their ability to form gels and thicken liquid product. For instance, pectin, carrageenan and agar form gels under specific conditions, which opens up a myriad of applications and functionalities.
Types of Pharmaceutical Thickening Agents
A wide range of excipients can be used as thickening agents, with the most common being hydrocolloid gums and cellulose ethers.
Hydrocolloid Gums
The term ‘hydrocolloid’ is derived from two Greek words, ‘hydro = water, and ‘kolla’ = glue. Currently, the term hydrocolloid is used for macromolecular (polymers) hydrophilic substances (with affinity for water), and includes polysaccharides and proteins.
When dissolved or dispersed in water, hydrocolloids hydrate and swell (increase in volume) due to solvent molecules penetrating into void spaces that exist between polymer chains. Hydration is accompanied by an increase in viscosity.
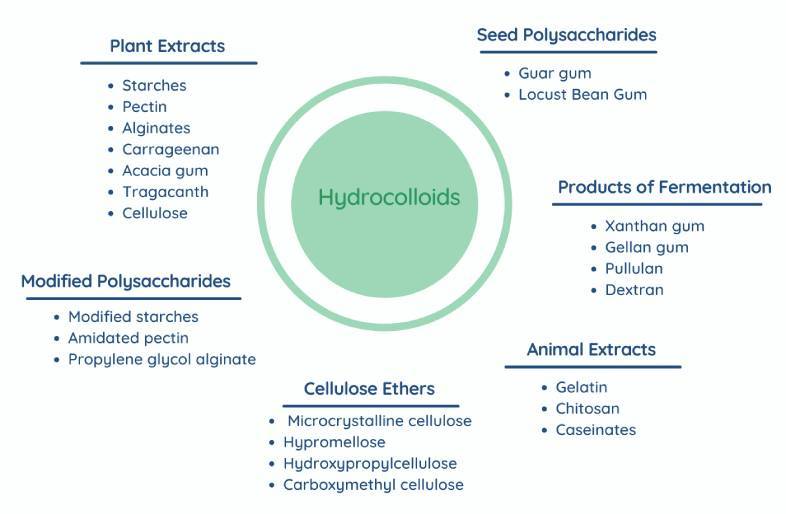
Hydrocolloids can be classified into four groups:
Plant-derived hydrocolloids: including pectin, agar, Alginates, acacia, tragacanth, Karaya gum, guar gum, starches, cellulose and locust bean.
Products of fermentation: including xanthan gum, dextran, gellan gum and pullulan.
Semi-synthetic hydrocolloids: including modified starches, cellulose ethers (Methylcellulose, Hypromellose, Ethylcellulose, Hydroxypropylcellulose and hydroxyethylcellulose), amidated pectin and propylene glycol alginate.
Animal derived hydrocolloids: such as gelatin, chitosan and caseinates.
Vinyl polymers
Vinyl polymers is a generic term given to high molecular weight synthetic polymers based on the vinyl groups: XCH=CHY. In the pharmaceutical field, the most important vinyl polymers are:
Water soluble polyvinylpyrrolidones: This group includes povidone and copovidone of molecular weights ranging from 2500 to 1250000 g/mol.
Ethylene-vinyl acetates: Ethylene vinyl acetates (EVAs) are copolymers of ethylene and vinyl acetate monomers produced by free radical polymerisation under high temperature and pressure.
Varying vinyl acetate composition impacts polymer properties such as melting point, crystallinity and polarity, solubility, transparency, hardness and compatibility with active ingredients.
EVAs are mainly applied in the formulation of transdermal drug devices and Class I – IV medical devices, where they are used as and/or incorporated into matrix formers, rate controlling membranes and backing layers. EVAs provide tackiness, bioadhesion and flexibility.
Polyvinyl alcohol: Polyvinyl alcohol (PVA) is a water-soluble and biodegradable polymer obtained by polymerisation of vinyl acetate followed by partial hydrolysis. Pharmaceutical grades typically exhibit a degree of hydrolysis of 88% and molecular weight that vary from 20 000 g/ml to over 100 000 g/mol.
Clays (Aluminium Silicates)
Clays are fine-grained naturally-occurring aluminium silicates with traces of other inorganic oxides. They typically consist of plate-like crystal habits. The most well-known clays in the pharmaceutical field are bentonite, hectorite and magnesium aluminium silicate.
Owing to their platy habits, aluminium silicate particle planes possess different surface charge characteristics and complex modes of particle-particle interaction, depending on concentration and pH, among other factors.
In suspension, hydrodynamic forces or the influence of electroviscous effects of clay solutions impacts rheology and viscosity of clay suspensions, much the same way as polymers dispersions.
Carbomers
Carbomer are a group of closely related synthetic, high molecular weight, nonlinear polymers of polyacrylic acids, cross-linked with a polyalkenyl polyether. They differ in molecular weight, polymerization solvent or side-groups and contain between 56 and 68% w/w carboxylic acid groups.
Carbomer are highly versatile and multifunctional excipients for oral (solid and liquids) and topical formulations. When hydrated and neutralised, they yield highly viscous gels with viscosities upwards of 40,000 mPa s for concentrations as low as 0.5% w/v.
If you’re interested in learning more about carbomers, we have prepared a quick reference guide which you access through this link:
Carbomers: Overview, key properties and formulating tips
Polyols, sugar and oligosaccharide
Concentrated solutions of sugars, oligosaccharides and polyols behave very much like high molecular weight polymer solutions owing to increased intermolecular attractions between sugar molecules (hydrogen bonds) and the solvent. When sheared, the interactions create a drag which manifests as an increase in viscosity.
This class of viscosity-increasing agents include liquid Sorbitol, liquid Maltitol, sucrose, fructose, dextrose, maltodextrin and Polydextrose.
Miscellaneous Thickening Agents
Polyethylene glycol and polyethylene oxide: Polyethylene glycol and polyethylene oxide are related non-ionic polymers formed by the reaction of ethylene oxide and water under pressure in the presence of a catalyst. Low molecular weight polyethylene glycol grades are liquids and used primarily as solvents and co-solvents in oral and topical formulations. Solid polyethylene glycol grades are used suspending agents or to adjust the viscosity and consistency of other suspending vehicles.
Silica: Also known as colloidal silicon dioxide, silica is an oxide of silicon with the chemical formula SiO2. Silica grades with a high specific surface area are used to thicken polar liquids thereby converting them into transparent gels. This effect can be used as stabilising strategy for emulsions and semisolid preparations.
Other materials which can be used for thickening formulations include:
- Silicones
- Glycerine
- Fatty acids
- Hard fats
Summary of Thickening Agents
Many pharmaceutical excipients are often added to formulations to increase the viscosity or thicken liquid formulations. This property can be utilised to improve product properties, including drug delivery, stabilisation, demulcent effects, and texturizing. The range of materials that serve this function is very broad, but typically includes hydrocolloid gums, synthetic polymers, sugars and polyol syrups and many other miscellaneous materials.