Therapeutics, in its broadest definition, is the use of interventions aimed at alleviating the effects of disease in humans or animals.
The constitutive substances that elicit therapeutic effects are known by different names, including active, active pharmaceutical ingredient, active principle, drug substance, medicinal agents or therapeutic substance.
This article provides a comprehensive definition of active pharmaceutical ingredients and as well as answers to common FAQs.
Definition of Active Pharmaceutical Ingredients
An active pharmaceutical ingredient (or API) is defined as the chemical, biological mineral or any other entity or component responsible for the therapeutic (pharmacological, physiological, physical, etc) effects in a product, such as:
- vaccine
- pharmaceutical (medicine)
- medical device
Active pharmaceutical ingredients are distinguishable from inactive pharmaceutical ingredients, commonly known as excipients or formulation aids. For a comparative discussion of what APIs are, click through this link for the World Health Organisation’s definition.
The European Medicines Agency, the US FDA and the International Conference on Harmonisation (Q7) all adopt the same definition of API as “any substance or mixture of substances intended to be used in the manufacture of drug (medicinal) products, and that, when used in the production of drug, becomes an active ingredient of the drug product.”
Many other terms are used interchangebly with active pharmaceutical ingredient. They include:
- active
- bulk pharmaceutical chemical
- drug substance
- active principle (especially for natural extracts)
- therapeutic ingredient
You may want to take note that health authorities add qualifiers to the definition of actives, namely, that a substance becomes an active ingredient in the drug product when it’s used in the production of the drug product, and, actives are intended to provide pharmacological activity or any other direct effect that is important in the diagnosis, cure, prevention, treatment or prevention of a disease condition, or to modify the structure or function of the body.
The reason is that there are many substances that are used beyond therapeutics. For instance:
- Insulin is also used in fermentation bioprocesses as well as an important medicine in diabetes
- Warfarin is an aid in pest control (culling agent of Vampire bats) as well as an anticoagulant
- Many other materials function as therapeutic substances as well as excipients. This list include simethicone which may be used as a processing aid or therapeutically as an anti-flatulent; docusate sodium is both an medicinal active (laxative) and a excipient (surfactant), and mannitol is used both as a filler in tablets and as a therapeutic substance in the treatment of glaucoma and kidney conditions.
Consider the fact that materials intended for use as pharmaceutical actives are subjected to very strict controls, with respect to quality controls during manufacturing, distribution and use, adding a qualifier to the definition allows regulators to apply the required standards to the relevant use category (API vs processing aid vs excipient), thus preventing dilution of standards.
A key attributes of active pharmaceutical ingredients is their ability to bind to receptors and elicit a physiological response that can also be advantageously used in the treatment of disease.
The image below is an illustration of a Tyrosine kinase receptor (TRK):
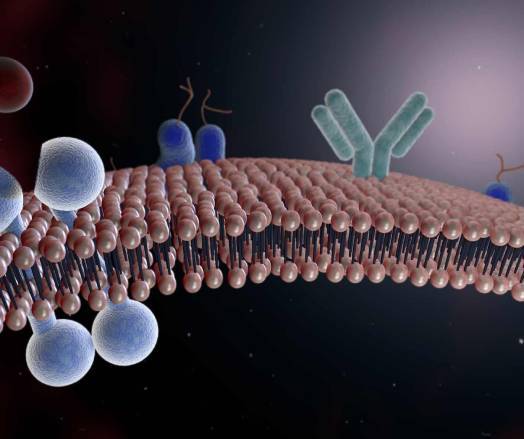
TRKs represent a widely studied class membrane receptors. They participate in many cellular functions, such as differentiation and apoptosis.
This has made them of particular interest in the search for anticancer agents, with more than 20 chemical agents successfully developed into therapeutic substances.
One of these is the active pharmaceutical ingredient, Imatinib, which is marketed as GLIVEC.
To find out more about how active pharmaceutical ingredients differ from excipients click on this link: Excipients Explained: Definition, Types, Uses, and Regulatory Controls.
What are the common sources of active pharmaceutical ingredients?
On the basis of origin, active pharmaceutical ingredients can be divided into four main categories as follows:
1. Synthetic organic chemistry as a source of drug substances
This group mainly includes small chemical substances, typically with a molecular weight of under 500 Daltons. The largest category of drug substances in use today are synthetic organic substances.
Examples of APIs obtained via synthetic organic chemistry include paracetamol (Panadol® – Glaxo) and artovastatin (Liptor® – Pfizer).
With better technology, it is also now possible to synthesise peptides having up to 20 amino acid residues in the laboratory for use as drug substances. Synthetic peptides include Somatostatin, Calcitonin (Fortical – )and Vasopressin (VASOSTRICT – Parpharm)
2. Natural organic molecules as sources of drug substances
From the time immemorial, humans have resorted to the nature as a source of therapeutic substances. This remains the case today.
For example, many antibiotics such as erythromycin and anticancer agents like paclitaxel (ABRAXANE® – BMS) are isolated from nature.
3. Recombinant DNA technology as a source of drug substances
Simply put, recombinant DNA technology is the process of altering gene of an organism and using the change to produce a biological molecule such as a large protein or chemical compound.
In just over a period of 40 years, recombinant DNA technology has grown to become one of the main sources of new drug substances today. Many important actives such as Human Insulin, Human Albumin, Erythropoietin and Human Growth Hormone are obtained this way, as are many vaccines and monoclonal antibodies.
4. Whole or Extractions from natural sources (animals)
There are still many therapeutic substances that can only be obtained from natural sources either as whole organisms or extracts from organisms.
Examples of these include blood and plasma, attenuated or live viruses used in vaccines and human immunoglobulins. The same applies to cells, tissues and organs used various in biotechnology modalities.
The table below summarises the main types of active pharmaceutical ingredients arranged by their source or origin:
| Active Type | Source | Examples |
|---|---|---|
| Conventional small-molecule drugs | Synthetic organic chemistry | Paracetamol, Ibuprofen, Imatinib |
| Conventional small-molecule drugs | Natural products | Paclitaxel, Aminoglycoside antibiotics, Opiates & Ciclosporin |
| Conventional small-molecule drugs | Semisynthetic compounds (derived from nature and modified in the laboratory) | Penicillins (Ampicillin) & Simvastatin |
| Peptides & proteins | Synthetic | Vasopressin & Somatostatin |
| Peptides & proteins | Extracted from natural sources (human, animal or microbial) | Insulin, Growth hormone & Human у-globulin |
| Peptides & proteins | Recombinant DNA technology | Insulin, Erythropoeitin, TNF-alpha |
| Antibodies | Animal antisera & human immunoglobulins | Snake & Spider venoms & HNIG |
| Antibodies | Monoclonal antibodies | Rituximab, Trastuzumab, etc |
| Enzymes | Recombinant DNA technology | Dornase, Galactosidase |
| Vaccines | Whole organisms (live or attenuated) | Smallpox, TB, tetanus, etc vaccines |
| Vaccines | Antigen-based vaccines produced by recombinant DNA technology | Several |
| DNA & mRNA products | Recombinant DNA technology | Patisiran |
| Cells | Human donor and engineered cells | Various stem cell therapies |
| Tissues | Human & animal donors & engineered tissues | Various |
| Organs | Human donors | Transplant surgery |
Irrespective of the type of drug substance, the process of isolating, preparing and purifying active ingredients is highly involved, and requires several painstaking steps.
In situation of high demand, this step-wise approach may not suit market requirements, for example, where the therapeutic good is in very high demand (Covid 19 vaccines?).
In recent decades, the pharmaceutical industry has sought to introduce technology aimed at improving synthetic yields of actives. When successfully applied, these technologies often result in major improvements in output over traditional processes.
Some technologies, though, promise much and deliver little. Click here to read about some of the technologies that promised much but have so far failed to improve drug discovery and development.
How are active pharmaceutical ingredients regulated?
EU legislation
The standards, requirements, and procedures that active pharmaceutical ingredients must meet before they can be used in marketing authorisations are primarily laid down in Directive 2001/83/EC, Regulation (EC) No 726/2004 and Directive 2011/62/EU. These regulations also set rules for the manufacture, distribution, and sale or advertising of medicinal products.
United States
In the United States, drug substances are controlled under the Federal Food, Drug, and Cosmetic Act of 1938 and subsequent amending statutes (which are all codified into Title 21 Chapter 9 of the United States Code). This law sets quality standards for drugs and medical devices manufactured and sold in the United States and provides for federal oversight and enforcement of these standards.
Did you know?
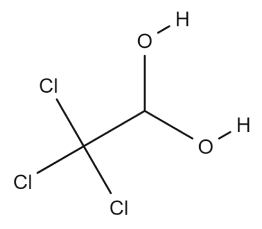
Did you know that the first synthetic active pharmaceutical ingredient is Chloral hydrate? It was synthesized by Justin Liebig in 1832 and introduced into medicine in 1869 as a sedative hypnotic. Although its use has declined, Chloral hydrate remains in use in some countries, particularly as a sedative for children.
Further Reading
- Nusim, S. (Ed.). (2010). Active Pharmaceutical Ingredients: Development, Manufacturing, and Regulation, Second Edition (2nd ed.). CRC Press. https://doi.org/10.3109/9781439803394