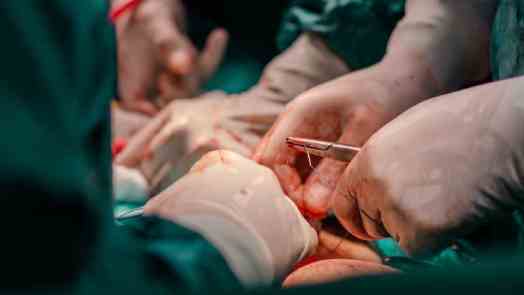
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New York City managed to attach a pig kidney to a human patient. The kidney functioned normally for 54 hours.
Last week, a team of surgeons in New York City were to able to successfully attach a pig kidney to a human patient and watch the organ function normally for a whole 54 hours. While procedures of this kind are not new in nonhuman primates, it is the first time that a pig kidney has been transplanted into humans and not been immediately rejected.
The process of transplanting living cells, tissues or organs from one species into another is what scientists call xenotransplantation. However, owing to genetic differences between species, past xenotransplantation efforts have not been successful, leading to immediate organ rejection by the human immune system.
The breakthrough procedure, which was announced at a news conference and widely reported in the media on October 21, represents a giant step towards the aim of increasing availability of life-saving organs for transplantation. Waiting list for donated organs around the world are in the millions, and demand is not expected to drop anytime soon despite a rise in organ donation registrations.
Speaking on condition of anonymity, a nephrologist at Abbott Northwestern Hospital, Minneapolis, MN said the fact that transplant survived three days with full function and no signs of rejection was an “incredible achievement,” and gives fresh confidence that “patients will have access to additional sources of organ for transplantation in the near future”.
However, several years of more of research, clinical trials and regulatory scrutiny are required before we can start to see pig kidneys on surgical tables.
In a press release, Robert Montgomery, MD and chair of the department of surgery at NYU Langone and director of the NYU Langone Transplant Institute, noted that the future of this work is not limited to kidneys.
“Transplanting hearts from a genetically engineered pig may be the next big milestone,” he said. “This is an extraordinary moment that should be celebrated — not as the end of the road, but the beginning. There is more work to do to make xenotransplantation an everyday reality.”
Why pigs?
In the quest to address the chronic shortage of organs, scientists have long sought the use animal organs. Pigs have emerged as an interesting choice because their organs are anatomically similar to those of humans, and they can be easily bred in a highly controlled manner.
However, it is more that ease of breeding. In the mid-20th century, xenotransplantation scientists noticed that transplanted animal organs quickly turned black, a phenomenon known as hyperacute rejection.
As knowledge has improved, scientists have been able to use genetic engineering to overcome some of these challenges. For example, it was found that aggressive immune responses seen after a pig xenotransplant was due to antibodies detecting alpha-gal, a sugar moiety found on porcine vasculature.
Disabling the gene that codes for alpha-gal was key to addressing hyperacute rejection of pig organs. Until now, a test of this sort of transplant hadn’t been done successfully in humans.
So what did the New York University Langone Health team do?
In order to overcome the many ethical hurdles of performing such an operations in humans the surgical team approached the family of a woman with brain stem death kept alive on a ventilator. Although the woman was an organ donor, her organs were not suitable for donation.
Over a period of several hours, the surgical team worked to attach the pig kidney, which had been genetically engineered to remove the alpha-gal sugar to blood vessels, in the upper leg of the patient. The kidney was kept outside of the body so the team could assess its function in real time.
In order to improve chances of acceptance, the team also transplanted the animal’s thymus gland, which aids the education of the immune system to recognize the kidney as part of the body. The patient was also given specific drugs that suppress the immune system.
Within minutes, the kidney started producing large amounts of urine and showed other signs of normal functioning. The pig kidney functioned just like a human kidney transplant. The research team stopped monitoring at 54 hours in line with IRB ethical guidance.
The patient was taken off life support after the procedure.
What’s next for these sort of transplants?
To survive 54 hours represents a significant development but to become mainstream, animal kidneys will need to survive for years not days. For this to happen, researchers will need to show that these organs can withstand immune system attacks for years in the human body.
A key aspect of this journal is to show that transplants are safe in the long-term and obtain approval from health authorities, including the U.S. FDA and the European Medicines Agency.
Are there any ethical concerns about breeding pigs for organ harvesting?
Using any animal for the sole benefit of humans raises important ethical questions. Advocates for xenotransplantation argue the potential benefits of expanding the organ supply are worth any potential harm done to animals.
The jury is still out on how acceptable the idea of breeding millions of pigs in order to harvest organs for human transplantation is.
PETA (People for the Ethical Treatment of Animals), a campaigning organisation against the use of animals in research, contests the whole idea that we should consign animals as sources of spare parts for humans (see their statement through this link).
Questions or comments on this article? E-mail us at editor@pharmacentral.com.
Sources
NYU Langone Health. Progress in xenotransplantation opens door to new supply of critically needed organs. Published online October 21, 2021.