Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Microcrystalline cellulose is an excipient made from purified, partially depolymerized cellulose prepared by acid hydrolysis of alpha-cellulose obtained from high grade wood pulp. Pharmaceutical excipient grade Microcrystalline Cellulose is available in different particle sizes and moisture grades, including speciality grades such as Silicified microcrystalline cellulose. It is supplied as a white, odourless, tasteless, crystalline powder composed of porous particles.
Pharmacopoeial Compliance: USP-NF; Ph.Eur; JP, IP, FCC
Synonyms & Trade Names: Microcrystalline Cellululose; Cellulose, Microcrystalline; Crystalline Cellulose; MCC; AVICEL®; EMCOCEL®; MICROCEL®; COMPRECEL®; E460; COELUS KG®; PHARMACEL®; FIBROCEL®
Uses & Applications: Filler, Binder and Compression Aid in Tablets and Capsules

Microcrystalline Cellulose is a purified, partially depolymerized cellulose-based pharmaceutical excipient and an approved food additive. It is derived from cellulose, and its chemical structure corresponds to the chemical structure of native cellulose. Pharmaceutical grade material is supplied as a white, freely flowing fibrous powder.
Cellulose, one of the most abundant biopolymers on earth and the main structural scaffold for plant cell walls, consists of thousands of β-D-glucose (C6H12O6) units linked by glycosidic bonds to form linear chains. Individual cellulose chains pack tightly and close to each other, a process that is enabled by inter and intramolecular hydrogen bonds and van der Waals forces. This leads to the creation of amorphous and crystalline regions within cellulose.
Crystalline regions in cellulose can be isolated following chemical and/or mechanical treatment of cellulose to produce various functional materials in the form of cellulose crystals. In the case of Microcrystalline cellulose, cellulose is treated with mineral acids, followed by purification and spray-drying, and optionally, milling. This is illustrated in the following scheme:
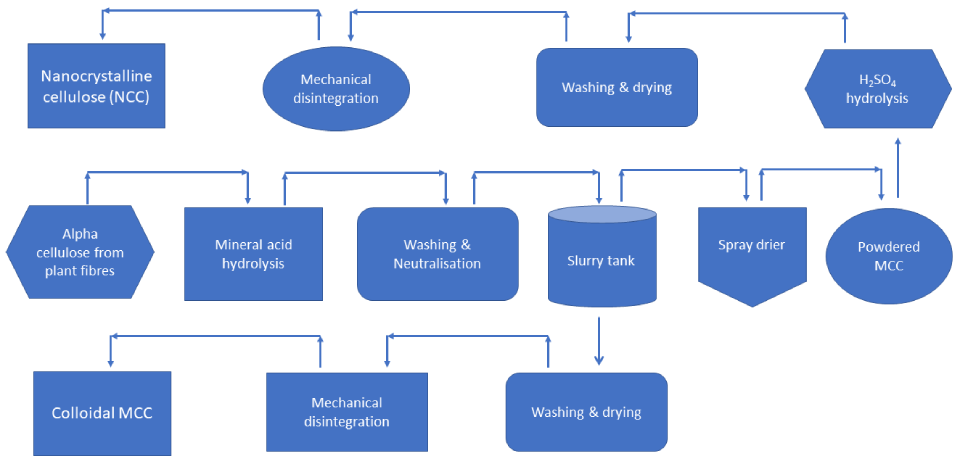
Microcrystalline cellulose possesses unique physicochemical properties, such as amphiphilicity, high crystallinity (approx., 70%), higher surface area, unique optical and hydration properties, special morphology, biocompatibility, and mechanical properties (plasticity), giving it wide application scope across multiple processing industries.
A number of these properties, especially particle size and moisture content, can be modified by varying the spray drying conditions. Milling may be undertaken to produce finer grades (<50 µm). Where higher bulk density grades are availed, these result from using specific wood pulps.
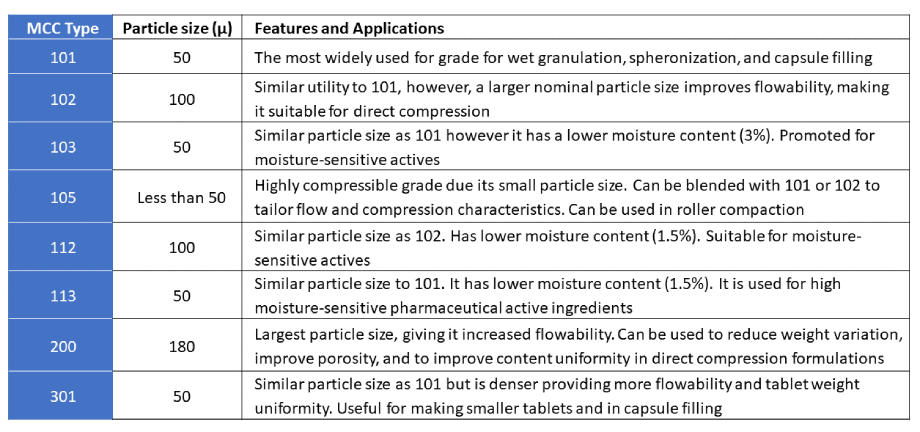
The table below shows the different grades of microcrystalline cellulose that are available commercially from different manufacturers:
Since its discovery in 1955 and subsequent commercialisation under Avicel by the FMC Corporation (later acquired by and until recently, part of Dupont), Microcrystalline cellulose remains as popular as ever, consistently emerging as the number 1 most used excipient in pharmaceutical and dietary supplements. The current estimates from researchers put the total global market to be worth in excess of US 1billion in 2020. North America is the single largest manufacturer of microcrystalline cellulose, followed by China.

| Chemical Name | 4-O-[(1S)-hexopyranosyl]-D-glycero-hexopyranose |
| CAS Registry Number | [9004-34-61] |
| Empirical Formula | (C6H10O5)n |
| Molecular Weight | Approx. 36 000 where n = approx. 220 |
| EINECS Number | 232-674-9 |
| UNII Code (FDA) | OP1R32D61U |
Microcrystalline cellulose is an approved excipient for use in oral and pharmaceutical products. It is listed in all major pharmacopeia, including the following:
Microcrystalline cellulose is also GRAS listed and accepted for use as a food additive in Europe and the United States. It is also included in the FDA Inactive Ingredients Database for:
However, Microcrystalline cellulose is not approved for parenteral use.
| Form | Solid, powder |
| Appearance | White crystalline powder |
| Angle of repose | Between 30 and 50 depending on grade |
| Density (bulk) | 0.28 – 0.36 g/ml |
| Flowability | 1.4 g/s (EMCOCEL® 90M) |
| Melting point (chars at) | 260-2700C |
| Moisture content | Hygroscopic. Typically less than 5% |
| Particle size distribution | Typical mean particle size is 20—200 µm |
| Solubility | Slightly soluble in 5% w/v sodium hydroxide solution; practically insoluble in water, dilute acids, and most organic solvents |
| Specific surface area | 0.78 – 1.4 m2/g |
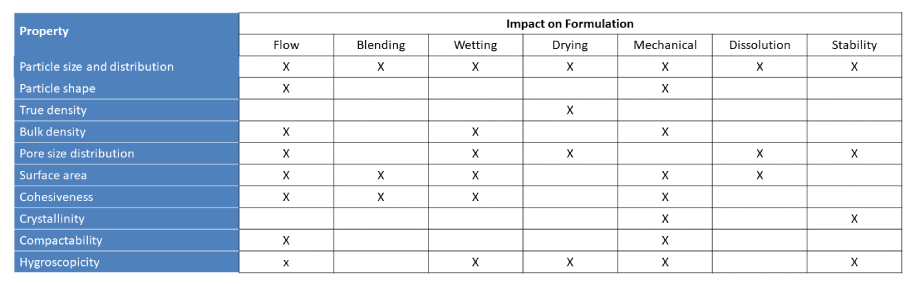
Key properties that affect the performance of microcrystalline cellulose
The role of different properties on material performance and processing behaviour is widely reported in the literature. The roles of a number of these properties is summarised in below:
Hygroscopicity/moisture content: Microcrystalline cellulose is moderately hygroscopic, absorbing approximately 5% moisture at equilibrium under ambient conditions. Low moisture grades are commercially available, having equilibrium moisture contents as low as 1.5%. Moisture content impacts compaction properties, tensile strength and plasticity. An increase in moisture content reduces compaction pressure required to achieve a given compact strength since water content enhances intermolecular interactions, and hence, particle bonding.
Particle size: Decreasing particle size improves the compactibility of microcrystalline cellulose due to the enhanced cohesiveness and greater particle-particle interactions. However, particle size has a negative impact on flowability. As a result, particle size impact hardness, friability, disintegration, and active content uniformity.
Particle morphology: Morphology refers to form and shape of particles, and is described in terms of length and width. Needle-shaped particles which are also fibrous (high length to width ratio) are cohesive and produce higher strength tablet compacts compared with spherical, non-fibrous particles.
Crystallinity: Microcrystalline cellulose has an estimated average crystallinity of ≈70%. Crystallinity depends on the quality of the wood pulp rather than processing conditions. Higher crystallinity leads to lower moisture content, and particle brittleness (Mohr hardness).
Bulk density: Low bulk density grades exhibit higher dilutive capacities without greatly affecting compressibility, which improves tabletability of low dose formulations.
| USP-NF | Ph.Eur | J.P | |
| Official name | Microcrystalline cellulose | Cellulose, Microcrystalline | Microcrystalline cellulose |
| Authorised use | Excipient | Excipient | Excipient |
| Definition | specified | specified | specified |
| Identification | specified | specified | specified |
| Appearance | specified | specified | specified |
| pH | 6.0- 8.0 | ≤ 0.25% | ≤ 0.25% |
| Bulk density | specified | ≤ 0.05% | ≤ 0.05% |
| Apparent Viscosity | specified | ≤ 20ppm | ≤ 20ppm |
| Water-soluble substances | ≤ 0.25% | ≤ 7.0% | ≤ 7.0% |
| Ether-soluble substances | ≤ 0.05% | ≤ 0.10% | ≤ 0.10% |
| Heavy Metals | ≤ 20ppm | ≤ 0.10% | ≤ 0.10% |
| Loss on Drying | ≤ 7.0% | specified | specified |
| Sulphated Ash | ≤ 0.10% | 103 cfu/g
102 cfu/g |
103 cfu/g
102 cfu/g |
| Residue on ignition | ≤ 0.10% | specified | specified |
| Conductivity | specified | specified | specified |
| Microbial limits Aerobic Molds |
103 cfu/g
102 cfu/g |
||
| Solubility | specified | ||
| Particle size | specified | ||
| Assay | n/a | n/a | n/a |
| Labelling | specified | n/a | n/a |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Microcrystalline cellulose is an exceptional material and one of the most widely used excipients in the pharmaceutical field. Its used in capsules and tablets, both in wet granulation and direct compression processes, where it serves as a moisture adsorbent, tablet, and capsule diluent, and tablet disintegrant. It is also widely used as a supplementary formulation aid, primarily to enhance the formulation robustness when less compactable diluents and binders are used.
Application as a Filler – Diluent
Microcrystalline cellulose works very favourably as a filler-diluent in wet granulation and direct compression operations. The higher dilution capacity coupled with excellent compactibility allow the production of strong cores at low compression pressures.
When selected in wet granulation, the optimum level of use is between 5 and 20%, although higher levels have been successfully used. In roller compaction, Microcrystalline cellulose improves compaction during the ribbon phase, enabling a trouble-free process while also preserving the plasticity of the compacts.
Application as a Binder
Microcrystalline cellulose can be used as a secondary binder in wet granulation and as a dry binder in direct compression formulations. The utility of microcrystalline cellulose as a binder stems from its moisture-absorbing properties, which permit controlled granule growth during wet granulation, while the fibrous nature of particles facilitates strong compact formation during direct compression.
Lubricity and Antiadherent Effects
Microcrystalline cellulose powders have a low coefficient of friction meaning that formulations where it is used exhibit lower than average ejection forces and adhesion to tolling during tabletting.
Disintegration and Dissolution Enhancement
Microcrystalline cellulose enhances the disintegration of tablets owing to its strong wicking properties. When included in the formulation, it enhances tablet break-up as water ingresses into the core, leading to swelling and/or deformation.
Spheronisation-Extrusion Aid
Microcrystalline cellulose is the excipient that forms the basis for controlled pellet formation during extrusion-spheronization. It offers the required rheological properties that are critical to wet mass blending, agglomeration, breaking and rounding, and ultimately, the quality of the final product.
Mouthfeel in Chewable tablets
A number of specialty grades of Microcrystalline cellulose, such as Avicel® PH-113, can provide a creamy and elegant organoleptic effects for chewable and buccal tablet formulations.
Applications in Topical products
Specialty and co-processed grades of microcrystalline cellulose are used in topical formulations as diluents, lubricants, stabilisers, and thickeners. Avicel® CE, Avicel® RC and Avicel® CL are grades of Microcrystalline cellulose from Dupont. They are respectively co-processed with Guar gum, and Sodium carboxymethylcellulose, and can be used in topical formulations to provide enhanced skin feel and stabilisation of oil-in-water emulsions due to its ability to form thixotropic structures.
Application in Food and Cosmetic products
Microcrystalline cellulose is also used within foods products (as a texturiser) and in cosmetics where it can substitute synthetic thickeners.
Beneficial features of Microcrystalline Cellulose
Several co-processed grades of Microcrystalline Cellulose are available, where additional excipients are added to produce a ready-to-use excipient system. Examples of these grade include:
Microcrystalline cellulose is GRAS Listed and considered a safe and non-hazardous material. It is approved for use in food products (as a bulking agent and to add crispness).
It is generally regarded as a relatively nontoxic and non-irritant material that is safe for human and animal consumption.
Both the World Health Organisation and the European Food Safety Agency regards Microcrystalline cellulose as having no safety concerns for the public when used in animal nutrition.
Upon ingestion, Microcrystalline cellulose is not absorbed into the bloodstream and therefore has little toxicological potential.
However, the consumption of large quantities of the material may have a laxative effect. This is unlikely to be a problem when Microcrystalline cellulose is consumed as part of a medical product where it is used as an excipient in the formulation.
You should observe appropriate safeguards when handling Microcrystalline cellulose. Wear gloves, eye protection and use a dust mask.
The UK Health & Safety Executive has set workplace exposure limits at 10 mg/m3 long-term (8 hr TWA) for total inhaled dust and 4 mg/m3 for respirable dust. The short-term limit for total inhalable dust is 20 mg/m3.
Although Microcrystalline cellulose is a stable material, being hygroscopic, it can absorb a significant amount of moisture from the atmosphere. Therefore, the bulk material should be stored in a well-closed container in a cool, dry place. Typical shelf is 3 years, extendable for another 12 months depending on handling and storage.
Microcrystalline Cellulose is sourced from sustainable wood pulp. As a biodegradable, natural substance, it is considered safe for the environment with no long-term impact on ecology or marine life. Microcrystalline Cellulose excipient grade achieved a total score of 80/100 by the Excipients Forum Sustainable Chemistry™ Score.
Other manufacturers of Microcrystalline Cellulose excipient grade include Mingtai Chemical Co. (Taiwan), Chemfield Cellulose Pvt (India), and Asahi Kasei (Japan). Asahi Kasei also manufacturers pharmaceutical-grade non-pareils based on Microcrystalline cellulose, a unique application.
Microcrystalline cellulose is a type of cellulose, which is a naturally-occurring large molecular weight polysaccharide polymer found in plants. To prepare microcrystalline cellulose, the fibrous parts of certain types of wood are treated with dilute acid under a slightly high temperature (>100 oC) and pressure. In the presence of acid and heat, the cellulose polymers break up into shorter chains known as microcrystals. After the acid is neutralised and other impurities are removed, the material is carefully dried to produce a white, fibrous, tasteless and odourless powder known as microcrystalline cellulose. What are the specific sources of microcrystalline cellulose? The main plants that are used for preparing microcrystalline cellulose are conifers. Cotton and bamboo are also possible cellulose sources. However, pharmaceutical grade microcrystalline cellulose requires a higher quality pulp, therefore, for this grade of microcrystalline cellulose, only wood pulp is utilised.
Microcrystalline cellulose was discovered in 1955 in the United States and commercialized under the brand name Avicel® by FMC Corporation (which is now part of Dupont). It was registered as a pharmaceutical additive in 1966. Within the pharmaceutical field, scientists use microcrystalline as a binding agent, a bulking agent and as a general formulation aid to improve the integrity of tablets and capsules. It may also be used in topical products but this is rarely done. Within the food industry, microcrystalline is the most popular additive, both as a base material and a functional material. It works as a stabiliser, emulsifier, texturiser and anti-caking agent. It’s added to bakery, confectionery and certain dairy products. Microcrystalline cellulose is also used in the paint sector, specifically as a stabiliser and thickening agent for water-based paintings. In cosmetics, it functions as a filler and emulsifying agent, especially for oily ingredients. These are just a selection of some of the uses of microcrystalline cellulose.
Microcrystalline cellulose poses no risk to humans when used as an excipient in medicines or as an additive in food products. It has been assessed by many agencies, including the World Health Organisation, the US FDA and the European Food Safety Agency and found to be a safe and non-hazardous material. It is generally regarded as a nontoxic and non-irritant material that is safe for human and animal consumption. Extensive studies done by the World Health Organisation (using radio-labelled microcrystalline cellulose) showed that when it is ingested, microcrystalline cellulose passes through the digestive system without getting absorbed. This does not mean that consumption of large quantities is not without harm, though. There are reports showing that it has a laxative effect (increased bowel movements) when used in large quantities.
Microcrystalline cellulose is an inert material. Microcrystalline cellulose was evaluated by the World Health Organisation and it was determined that on the basis of the toxicological data from humans and animals, there is no evidence that the ingestion of microcrystalline cellulose cause toxic effects in humans when used in medicines or foods. Therefore, at the levels it is used in medicines and foods, microcrystalline cellulose poses no harm and has no adverse effects when taken orally. The US FDA and European Food Safety Agency have not set an allowable daily intake for microcrystalline cellulose. However, if consumed in high doses (>10g per day), microcrystalline cellulose may cause increased bowel movements. These levels are much higher than the averages typically consumed through medicines (0.3g per person per day) and food (1.67g per person per day).
Although microcrystalline cellulose is hygroscopic (absorbs water from its surroundings), it is actually insoluble in water, alcohols, other organic solvents and acids. However, it swells when it comes into contact with water.
Since microcrystalline cellulose is available in different grades of differing particle sizes and moisture grades each grade has different properties and applications. Here are some of the properties:
| Angle of repose | 30 - 50o depending on grade |
| Density (bulk) | 0.28 - 0.36 g/cm3 |
| Flowability | 1.4 g/s (Emcocel 90M) |
| Melting point Chars at. | 260-2700C |
| Moisture content | Hygroscopic. Typically less than 5% w/w |
| Particle size distribution | Typical mean particle size is 20—200 µm. |
| Solubility | Slightly soluble in 5% w/v sodium hydroxide solution; practically insoluble in water, dilute acids, and most organic solvents. |
| Specific surface area | 0.78 - 1.4 m2/g |
Microcrystalline cellulose is listed in all the major pharmacopoeias, including the United States Pharmacopoeia, the European Pharmacopoeia and the Japanese Pharmacopoeia. The different specifications are shown below:
| Test | Specification | Reference |
| Identification | White or almost white, granular powder | USP-NF/PhEur |
| Appearance | + | USP-NF/PhEur |
| pH | 6.0- 8.0 | USP-NF/ PhEur |
| Bulk density | + | USP-NF/PhEur |
| Apparent Viscosity | + | USP-NF/PhEur |
| Water-soluble substances | ≤0.25% | USP-NF/PhEur |
| Ether-soluble substances | ≤0.05% | |
| Heavy Metals | ≤20ppm | USP-NF/PhEur |
| Loss on Drying | ≤7.0% | USP-NF/PhEur |
| Sulphated Ash | ≤0.10% | USP-NF/PhEur |
| Residue on ignition | ≤0.10% | USP-NF/PhEur |
| Conductivity | + | USP-NF/PhEur |
|
Microbial limits
Aerobic Molds |
103 cfu/g 102 cfu/g |
USP-NF/PhEur |
| Solubility | + | USP-NF/PhEur |
| Particle size | + | USP-NF/PhEur |
Microcrystalline cellulose is obtained from cellulose obtained from wood pulp. Cellulose is the most abundant natural polymer on earth, with an annual biomass production of 50 billion tons, so there’s plenty to go around! Microcrystalline is therefore renewable. It is also non-toxic and biodegradable meaning that it does not affect aquatic life through accumulation.However, the increased interest in microcrystalline cellulose could put pressure on land for more wood pulp, which could lead to other issues. Scientists are looking for alternative ways to obtain microcrystalline cellulose to meet the increase in demand for this material.
Microcrystalline cellulose for pharmaceutical use is mainly produced by the following companies:
Microcrystalline
cellulose is 100% derived from plants. It is gluten free. However, it may not
necessarily meet the standards for Vegan status as this is dependent on how it
is processed. Most manufacturers serving the pharmaceutical sector declare it
as Vegan. For other producers, it is important to cross-check with them
directly.
Opinion on keto
status is controversial; however microcrystalline cellulose is not absorbed but
maybe a source of fibre.
Microcrystalline
cellulose is non-GMO.
This short video summarizes the features and
properties of microcrystalline cellulose and its uses in pharmaceutical
formulations.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.