Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
[bsa_pro_ad_space id=5]
Colloidal silicon dioxide is amorphous silica (oxide of silicon) prepared synthetically by the vapour-phase hydrolysis of a silicon compound. Colloidal silicon dioxide has the chemical formula SiO2 but is distinct from other types of silica, such as amorphous or crystalline silica, that exist naturally or otherwise such as silica gel or precipitated silica. Colloidal silicon dioxide is supplied as a white or almost white, light, fluffy, and extremely fine powder.
Pharmacopoeia Conformity: USP-NF; Ph. Eur; JP; IP; FCC
Synonyms & Trade Names: Silica Colloidal Anhydrous; Pyrogenic Silica; Fumed Silica; Colloidal Anhydrous Silicon Dioxide; Light Anhydrous Silicic Acid; Synthetic Amorphous Silica; SAS; Fumed Silicon Dioxide; Silicic Anhydride; Silicon Dioxide Colloidal; Hydrophilic Silica; E551; AEROSIL®; Cab-O-Sil®; Wacker HDK; AEROPERL® 300
Uses & Applications: Glidant; Anticaking Agent; Emulsion Stabilizer; Suspending Agent; Thermal Stabilizer; Viscosity-Increasing Agent; Desiccant, and Solubility-Enhancer

Colloidal silicon dioxide Anhydrous is a highly pure, very fine amorphous silicon dioxide having primary particles in the nano-scale range. It is obtained through chemical synthesis using a suitable silicon compound precursor, such as silicon tetrachloride, typically at temperatures >2000 oC. It is for this reason that it is also referred to as “fumed” or “pyrogenic” amorphous silicon dioxide. It is distinct from other types of silicon dioxide, whether natural or synthetic, amorphous or crystalline. In different pharmacopoeia, Anhydrous colloidal silicon dioxide is described as a fine, white, amorphous powder consisting of sub-microscopic particles (about 15 nm). It contains between 99.0 – 105 % of SiO2. It is insoluble in water, organic solvents and mineral acids.
Silicon and oxygen are the two most abundant elements in the earth’s crust. In nature, silicon almost always exists in combination with oxygen, either as free silica (SiO2), in conjunction with other elements (for instance, in silicates, which are the main minerals in rocks and soil), or as combined silica (SiO3). The different silicon compounds have substantially different chemical properties, applications, and hazards.
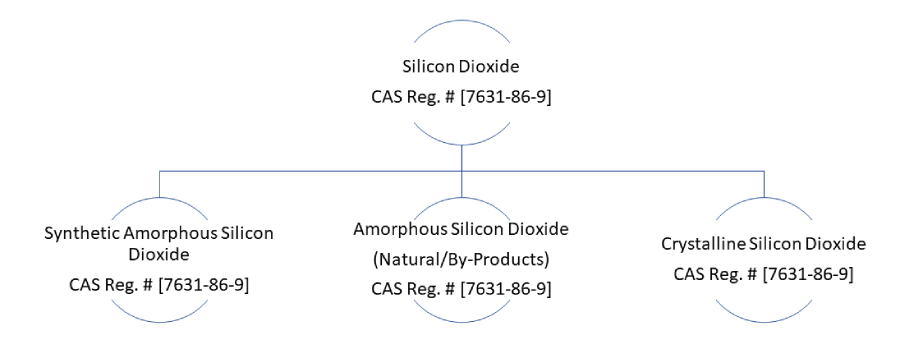
Free silica (Silicon dioxide) is a hard, low-reactivity, colourless substance that occurs naturally in rocks and minerals or can also be industrially produced in the form of synthetic amorphous silica. All forms of Silica, whether natural, synthetic, crystalline, cryptocrystalline or amorphous, are assigned a single CAS Registry number [7631-86-9]. For convenience, the different forms of Silica can be divided into three main groups as shown in the chart below:
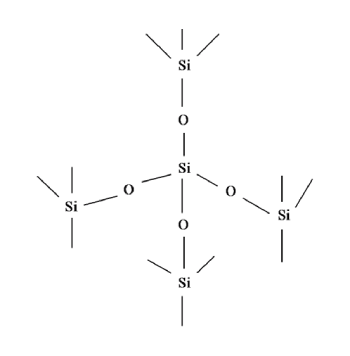
At the fundamental level of Silica’s chemical properties is the Silica tetrahedron (SiO4), which consists of a central Silicon cation covalently bonded to four oxygen atoms, arranged in the shape of a tetrahedron. Silica tetrahedra may be linked and arranged in a variety of ways, from simple to complex three-dimensional frameworks. Crystalline forms of Silica exhibit a highly ordered crystal lattice, determined by the ordered arrangement of the Silica tetrahedra. Amorphous forms, on the other hand, have random, disordered lattices. The orientation of the bonds is random, and there is no long-range periodicity.
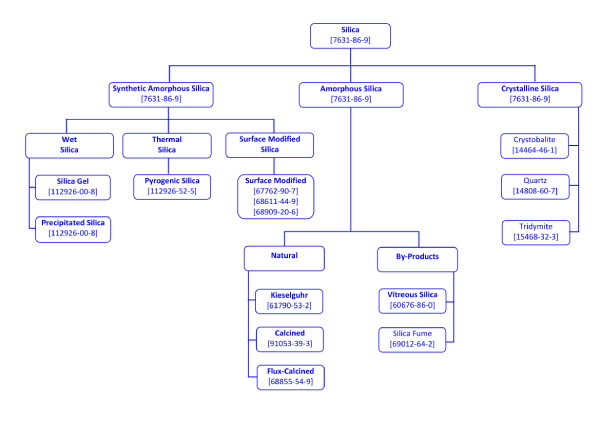
To differentiate between the different silica analogues, new CAS Registry numbers have been assigned. These are shown in the chart below:
![]()
Synthetic amorphous silicon dioxides are further divided into three main types, namely:
Even though they share the same chemical structure and synthetic origin, Synthetic silicas exhibit different properties, as briefly reviewed below:
Pyrogenic silicon dioxide is produced using a high-temperature process in which silicon tetrachloride is vaporised in an oxygen-hydrogen atmosphere according to the following chemical reaction:
SiCl4 + 2H2 + O2 -> SiO2 + 4HCl
The raw materials used in the silica production process are all inorganic and very pure. As a result, the synthesis produces only hydrochloric acid (which is easily removed) and silica in very high states of purity (typically > 99.9%). Silicon dioxide produced pyrogenically exists in the form of chain-like, branched aggregates, giving rise to a fluffy, light powder. (The term “fume” alludes to the method of manufacture, which involves the use of a flame.) Varying processing conditions allows the production of silica products with different specific surface areas, typically between 50 and 400 m2/g.
The pyrogenic method for producing Silica was invented in 1941 by Harry Klopfer, a scientist at Degussa (now part of Evonik AG). This method is what is still used by Evonik (for the production of pyrogenic silica marketed under the AEROSIL® brand name) and Cabot Corporation (for the Cab-O-sil® fumed silica brand). Note that these silica grades can be used in their native (unprocessed) state. They can also be further processed (for example, spray drying, granulation, or surface chemical modification) to turn them into other technical silica grades.
Precipitated silicon dioxide is silica produced in an aqueous solution at temperatures >60 °C. In this process, sodium silicate (waterglass) undergoes controlled neutralisation with either concentrated sulphuric acid or hydrochloric acid. The Silica precipitates out as a slurry of (hydrated) silica, which is washed and filtered to remove by-products. It is then dried in hot air and milled or passed through a classifier.
Precipitated silicon dioxide has been known since the mid 17th century. It was not until the 1920s that its practical uses and industrial production were fully established. Currently, Precipitated silica is produced in volumes that are up to x10 greater than for Pyrogen silica. The method and conditions have been fine-tuned and now permit production of many types of synthetic silica that are structurally and characteristically different, including exhibiting internal pore volume/specific surface area, larger particle sizes, and water content.
3). Surface-Modified Silicon Dioxide
The Silica grades described thus far are available for use as in their native or unmodified state. These materials have freely accessible silanol groups (Si-OH) on the surfaces of Silica particles, rendering them hydrophilic. Frequently, it is desirable to have hydrophobic silica, i.e a product that repels water. Hydrophobicity can be achieved through a post-synthesis step in which the silanol groups are reacted with organic groups. The added organic groups are tightly bound to the surface (via covalent bonds) and are only broken via thermal decomposition.

Pharmaceutical-approved hydrophobic silica is produced by reacting hydrophilic silicon dioxide with dimethylchlorosilane immediately after the production of Silica particles in the hydrogen flame chamber. This process is also conducted at high temperatures and allows dimethysilyl groups to be bound irreversibly onto the surface of the silica via siloxane bonds. This produces a material that, while appearing identical to the precursor Silica, is very hydrophobic, repels water and does not absorb moisture from the environment.
“Colloidal” is used in reference to both pyrogenic and precipitated silica. It may be confused with Silica colloids, which are also obtained via the wet chemical route. Note that the International Union of Pure and Applied Chemistry (IUPAC) defines colloids as systems (dispersions) in which particles of colloidal size (1 nm–1000 nm) of any nature (solid, liquid, or gas) are dispersed in a continuous phase of different composition or state.
Thus, in the strictest sense, the term ‘colloidal silica’ applies to stable dispersions (or sols) consisting of discrete particles of amorphous silica having sizes of between 5 and 100 nm. These colloidal silicas are commercially available in the form of sols or dried powders (e.g., xerogels, dry precipitates, aerogels, or calcinated coarcervates). In a broad sense, however, many other forms of silica (other than wet or dry silica sols above) are colloidal on the grounds that they are composed of particles in a colloidal state of subdivision (1-1000 nm).
It is also worth noting that the silica particles and aggregates are self-supporting and stabilised dispersions of silica particles in a continuous air phase and are unaffected by gravitational forces. Finally, fumed silica is commonly referred to as ‘colloidal’ because the silica powders are made by condensing a silica precursor from a vapour phase. In this sense, fumed silica particles are dispersed in a gaseous phase during production process.
The SiO2 molecules in synthetic silicon dioxide do not exist in isolation. While the primary structure is the tetrahedron, consisting of one silicon atom bonded to four oxygen atoms, tetrahedrons arrange into networks. During the synthesis process, minute droplets of SiO2 initially aggregate into so-called nuclides, which combine stochastically into even larger particles, facilitated by weak physical interactions such as van der Waal’s forces.
Another important property of synthetic silica is its specific surface area. Fumed silicon dioxide, in particular, has only one surface, which is external and little or non-existent internal pore volume. Precipitated silica, on the other hand, is mesoporous and exhibits an internal surface. Generally, the higher the specific surface area, the greater the degree of agglomeration. It is these aggregates that partly contribute to the unique functionalities of amorphous silicon dioxide.
| Chemical Name | Silicon dioxide, chemically synthesised |
| CAS Registration Number | [112945-52-5] |
| Empirical Formula | SiO2 |
| Molecular weight | 60.08 |
| EC Number | 231-545-4 |
| UNII Code (FDA) | ETJ7Z6XBU4 |
Colloidal silicon dioxide is approved for use in pharmaceutical products as an excipient (oral, topical products and topical sprays but not for inhalation). It is listed in all the major pharmacopoeia, including the USP-NF, Ph.Eur, I.P, B.P, and J.P.E. A specification for Colloidal silicon dioxide is included in the Food Chemicals Codex (FCC). It has been used in pharmaceuticals, food products, and cosmetics for over 50 years.
Colloidal silicon dioxide is currently approved by the FDA, is GRAS listed and included in the FDA Inactive Ingredients Database. Both the European Union and the FDA have authorised the use of Colloidal silicon dioxide as a food additive and for food contact materials. In Europe, Colloidal silica is designated E551. The following regulatory schedules apply to colloidal silicon dioxide:
| Jurisdiction | Framework | Record | Comment |
| European Union | European Pharmacopoeia | Monograph – ‘Silica, Colloidal Anhydrous’ | |
| Food Additive | E551, as Silicon Dioxide | ||
| Animal Nutrition | E551a and E551b | ||
| United States of America | United States Pharmacopoeia | Monograph – ‘Colloidal Silicon Dioxide’ | |
| Food Additive and Contact Material | FCC specification – ‘Silicon Dioxide’ | Limits apply | |
| Animal Nutrition | 21 CFR.133 & 160 | Limits apply | |
| Japan | Japanese Pharmacopoeia | Monograph – ‘Colloidal Silicon Dioxide’ | |
| Animal Nutrition | Food Safety & Quality regulation |
Several grades of colloidal silicon dioxide are widely available, depending mainly on the method of production, and any subsequent processing changes that the material is subjected to. These changes, while not necessarily affecting the silica content, density, refractive index, colour, or amorphous form, do impact three key parameters, i.e:
As a result, different commercial grades of colloidal silicon dioxide, such as AEROSIL® (Evonik), Cab-O-Sil (Cabot Corporation) and Wacker HDK (Wacker-Chemie GmbH) may differ in these parameters. The table below provides a synopsis of the main physicochemical properties of importance.
| Physical form | Solid, powder |
| Appearance | White |
| Flowability | Fluffy, poorly flowing or free-flowing powder |
| Hygroscopicity | Colloidal silica is hygroscopic. Moisture content is dependent on grade and storage conditions |
| pH value | pH 3.5 – 5.5 (4% w/v aqueous dispersion) |
| Poured Density | 0.03 – 0.04 g/ml |
| Tapped Density | Dependent on grade |
| Melting point | 16000C |
| Particle size distribution | Primary particle size are in nano range (5 – 20 nm). However, most Silica exists in the form of loose agglomerates of 10 to ≥100µm. |
| Refractive index | 1.46 |
| Solubility | Practically insoluble in water, propylene glycol, glycerol, organic solvents, mineral oil, and most acids. Soluble in hot solutions of alkali hydroxide. |
| Relative density | 2.2 |
| BET Specific surface area | 50 – 600 m2/g depending on grade and supplier |
| USP-NF | Ph.Eur | J.P | I.P | |
| Name | Colloidal Silicon Dioxide | Silica, Colloidal Anhydrous | Light Anhydrous Silicic Acid | Silica, Colloidal Anhydrous |
| Authorised Uses | Excipient | Excipient | Excipient | Excipient |
| Definition | specified | specified | specified | specified |
| Characters | n/a | specified | n/a | specified |
| Identification | specified | specified | specified | specified |
| pH | 3.5-5.5 | 3.5-5.5 | n/a | 3.5-5.5 |
| Chlorides | na | ≤250 ppm | ≤0.011 % | ≤250 ppm |
| Aluminium | specified | n/a | n/a | n/a |
| Iron | n/a | n/a | ≤500 ppm | n/a |
| Calcium | specified | n/a | n/a | n/a |
| Arsenic | ≤ 8 ppm | n/a | ≤ 5 ppm | ≤8 ppm |
| Heavy Metals | na | ≤ 25 ppm | ≤ 40 ppm | ≤ 25 ppm |
| Loss on Ignition (%) | ≤ 2.0% | ≤ 5.0% | 12.0 % | ≤ 5.0 % |
| Loss on Drying (%) | ≤ 2.5% | n/a | 7.0 | n/a |
| Volume Test | n/a | n/a | n/a | ≤ 70 ml |
| Assay (%) | 99.0-100.5 | 99.0-100.5 | ≥98.0 | 99.0-100.5 |
| Labelling | Specified | n/a | n/a | n/a |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Colloidal silicon dioxide is one of the most widely used pharmaceutical excipients today. It is used in solid and liquid dosage forms, food products, and cosmetics and has been in use for over 60 years. Its usefulness stems from three key attributes:
Colloidal silicon dioxide is used as a carrier for liquids and semi-liquids or as free-flow agent in powdered products (pharmaceuticals, cosmetics, salts, and foods), particularly for substances that attract water molecules (hygroscopic) or cake, so that they flow better.
It also provides pastes and ointments with the desired consistency and prevents separation of the various formulation ingredients, making it a highly versatile material with multiple pharmaceutical and cosmetic applications. This versatility stems from its high absorptive capacity, ability to improve flow, and low dust content, as outlined in the table below:
| Application | Important Properties |
| Absorbent/desiccant | Porosity |
| Matting, gelling and structuring effects | Aggregate particle size |
| Toothpaste | Aggregate particle size |
| Elastomer reinforcement | Aggregate particle size |
| Free-flow, anti-caking & glidant | Porosity, aggregate particle size |
| Rheology control | Surface chemistry |
When applied to pharmaceutical products, the above-listed Silica attributes provide the following functionalities:
Thus, Colloidal silicon dioxide functions, in both solid and liquid dosage forms, as a:
In tablets, capsules, and granules, Colloidal silicon dioxide is popularly used as a glidant and anti-adherent, where it allows powder formulations to efficiently flow on high-speed tablet presses. The available grades and their uses are outlined in the table below:
| Product | Properties | Benefits |
| AEROSIL® 200 Pharma (Evonik)
CAB-O_SIL® M5 PD (Cabot) HDK® N20 Pharma (Wacker) |
Hydrophilic silica. 175-225 m2/g (BET Surface area). Untreated surface. | Traditional glidant for solid dosage forms. Suitable for formulating soft powder formulations. Thickener for oils |
| AEROSIL® 200 VV Pharma (Evonik)
HDK® N20P Pharma |
Hydrophilic, densified silica. 175-225 m2/g (BET Surface area). Untreated surface | Glidant and thickener applications but with reduced dusting and less volume requirements |
| AEROSIL® 300 Pharma (Evonik) | Hydrophilic silica. 270-330 m2/g (BET Surface area). Untreated | Ideal glidant for hygroscopic powders that exhibit particle-particle cohesiveness via liquid bridge formation |
Glidants permit maximization of throughput on high-speed machinery while also ensuring products meet quality and regulatory requirements for dose and content uniformity.
A key advantage of Colloidal silicon dioxide is that only a small quantity is needed to improve the flowability and compressibility of a powdered formulation, which yields strong tablets and higher content uniformity. For most formulations, standard hydrophilic Colloidal silicon dioxide grades are sufficient due to their ability to efficiently adsorb moisture and keep powders dry and free flowing (typical usage levels is 0.1-0.5%).
How Glidants Work
Several forces are at play when it comes to particle-particle interactions, and, thus, powder cohesiveness and flow. In general, these forces include but are not limited to van der Waals forces, electrostatic forces, liquid bridges, and physical entanglements. The smaller the particles, the greater the interactions, and thus, the more cohesive a powder is. This is the reason micronised powders do not flow well.
Colloidal silicon dioxide improves powder flow by counteracting the forces behind particle cohesion. This is achieved by adhering to the surface, increasing the distance, and reducing attractive forces (van der Waals forces and electrostatic attraction decrease with increasing distance between the particles).
In addition, the hydrophilic nature of Colloidal silicon dioxide enables it to attract and preferentially bind moisture, helping to eliminate liquid bridges between solid particles that hinder powder flow.
Finally, aggregates of Colloidal silicon dioxide occupy and fill voids, which eliminates irregularities on the particle surfaces, reducing particle-particle entanglements.
How to Use Colloidal Silica As a Glidant
Colloidal silicon dioxide is used in tablet coating processes due to its ability to improve throughput and economics. In standard coating processes involving powder layering, Colloidal silicon dioxide is used to facilitate powder build-up in the coating while also helping maintain pigments in suspension.
In acquiring good flowability (lubricity), the build-up powder distributes more evenly on the cores and dries much faster, which shortens the entire coating process considerably. Meanwhile, much stronger coats are obtained since the cores spend relatively shorter time periods in the coater. The high moisture binding capacity of Colloidal silicon dioxide also protects cores from over wetting.
Whether powder build-up is used, or only highly concentrated coating suspensions are used, Colloidal silicon dioxide is still useful for stabilising pigment suspension and improving a coating’s smoothness. In this regard, the recommended levels of Colloidal silicon dioxide for use in coating build-up are 10 to 15% w/w, while the levels for aiding pigment suspension are 0.5 to 2.0% w/w.
Colloidal silicon dioxide grades find utility in liquid formulations on account of their viscosity-modifying properties as well as their ability to prevent particle settling in disperse (suspensions) systems. Both effects are based on the ability of dispersed Colloidal silica particles to form a network of aggregates via hydrogen bridge linkage and/or van der Waals attractive forces in liquid media.
Thus, when the fumed silica is dispersed in a liquid, silanol groups interact with each other either directly or indirectly via the molecules in the liquid. This affinity is attributed to hydrogen bonding, and results in a reversible, three-dimensional lattice structure that manifests macroscopically as thickening.
When the system is mechanically sheared, such as through shaking, the structure is broken down, rendering the system more fluid as the viscosity drops. At rest, however, the lattice rebuilds, and the viscosity returns to what it was before, a phenomenon known as thixotropy.
Several nonpolar liquids, such as vegetable oils, liquid paraffin and isopropyl myristate, can be converted into spreadable gels with Colloidal silicon dioxide. Provided the liquid has a refractive index closer to that of the silicon dioxide (i.e., 1.48), generally transparent gels will be obtained.
A key feature of gels obtained with colloidal silica that makes them different from other types of gels (for instance, those obtained with hydrocolloid gums) is that their viscosities show little dependence on temperature. This makes them well-suited for formulations whose storage and temperature stabilities are critical.
Generally, the viscosity of obtained gels depends on the amount of silica added; that is, the more is added, the thicker the gel will be. Any standard silica grade can be used, although grades with much larger specific surface areas, such as Evonik’s AEROSIL® 300 Pharma are the most recommended. Densified grades are not used to thicken oils because they form larger, more stable agglomerates that are difficult to disperse.
How to Use Colloidal Silica to Thicken Oils
Colloidal silicon dioxide is an important ingredient in the formulation and production of suppositories. It allows formulation ingredients to be uniformly distributed throughout the suppository base.
In addition, it increases the softening point of the suppository base without changing its melting point, an important property for improving stability in warm climates. The consistency and mechanical stability of the finished suppository are also improved.
In the unlikely event that an active ingredient inadvertently reduces the melting point of the suppository base (especially solution suppositories), silica can be added to prevent this phenomenon. The recommended procedure is to initially “triturate” the offending ingredient with the colloidal silicon dioxide before adding it to the product.
Colloidal silicon dioxide can be used in membrane-controlled and matrix-controlled transdermal drug delivery systems as a gel former or to increase storage and thermal stability. It can also be used as a carrier or adsorbent for active ingredients and to improve incorporation or release characteristics.
The recommended concentrations of colloidal silica in transdermal drug delivery systems are generally between 1 and 5 % w/w. The silica should be dispersed in the medium that contains the active ingredient, polymer (adhesive) and/or other excipients.
Colloidal silicon dioxide is an effective excipient for stabilizing dispersions of solids in liquids and preventing the formation of hard sediments in liquid suspensions and aerosol formulations intended for topical (rather than inhalation) use.
Colloidal silicon dioxide allows suspended particles to remain in suspension long enough until they are deposited on the substrate, for instance, the skin, or in the case of coatings, the tablet core. The recommended usage levels are generally between 0.5 to 3.0% w/w.
Systemically acting drug products formulated as solid oral dosage forms necessarily require their active pharmaceutical ingredients (APIs) to dissolve in gastrointestinal juices before absorption into the bloodstream can occur. Therefore, APIs must first undergo dissolution in gastric juices and successfully permeate the lipid membrane of the intestinal wall. APIs that exhibit high solubility and high permeability generally have high bioavailability.
The importance of drug dissolution and permeability has been previously described by the Biopharmaceutics Classification System (BCS), originally proposed by Amidon, which groups APIs into four different classes according to their permeability and solubility in aqueous media, namely, BCS I, II, III and IV. Pharmaceutical scientists have used many different technologies to improve the solubility of products exhibiting poor solubility and/or permeability, including API modification, lipid formulations, solid dispersions, and speciality techniques (liposomes, prodrugs, etc).
Speciality granulated silica grades consisting of spherical particles in the range of 10–100 μm that feature a high internal surface (mesoporous) and high density can be used in the above-mentioned formulation strategies aimed at enhancing solubility and dissolution. An example of such mesoporous Colloidal silicon dioxide is AEROPERL® 300 Pharma (Evonik AG). AEROPERL® 300 Pharma, which like standard Colloidal silicon dioxide, is tested against the high-quality requirements of the “Silica Colloidal Anhydrous” (Ph. Eur) and “Colloidal Silicon Dioxide” (USP/NF) monographs.
Mesoporous Silica can be used to improve API solubility in a number of ways, as outlined below:
Moisture Activated Dry Granulation (MADG) is an alternative granulation technique that uses very small amounts of water (2-4% w/w) to initiate granule formation. Compared with traditional wet granulation, MADG does not involve the use of heat to dry granules and involves fewer process steps.
The process steps involved in MADG are shown below:
MADG was developed by Bristol Myers Squibb in the 1980s to address the challenges associated with wet granulation, including end-point determination, drying, milling, and moisture-instigated instability from hydrolysis.
A grade of mesoporous Colloidal silicon dioxide featuring a high specific surface area can be used as a moisture desiccant in MADG. The silica, together with other excipients, helps absorb and distribute the moisture, which yields uniform, free-flowing, and compactible granules.
Note that the aim of the MADG process is not to make large granules, but rather to agglomerate fines and bind the drug with excipients into freely flowing, compressible granules that can be tabletted without further processing.
As a result, MADG offers multiple advantages, including reduced energy costs, fewer processing steps, and reduced investment in machinery.
Colloidal silicon dioxide is a purely inorganic substance. The production process does not involve plant or animal based raw materials, nor does it involve organic solvents. It has been produced and utilised in medical and food products for over six decades and has been extensively studied by health authorities and many other scientific organisations.
For a long time, Colloidal silicon dioxide was incorrectly associated with crystalline forms of silica since the two materials shared the same name and chemical formula. Crystalline silicas have been shown to be responsible for many of the deleterious effects, such as silicosis. On the other hand, silicon dioxide grades that are both synthetic and amorphous in structure do not share the biologic hazards. They do not cause silicosis. However, they should not be used for drug delivery by inhalation or injection because of the risks of tissue reactions or the formation of granulomas.
Evidence obtained from the many studies done over several decades has led authorities to conclude that colloidal silicon dioxide has minimal adverse health effects from exposure, and is not harmful when administered orally or dermally. It is not irritating to skin and eyes and passes through the gastrointestinal tract without being assimilated in detectable amounts.
The European Union’s Scientific Committee on Food (SCF) concluded that silicon dioxide did not raise concerns with respect to genotoxicity. However, it should not be administered parenterally, because untoward tissue reactions or the formation of granulomas could occur.
Finally, colloidal silicon dioxide is inert toward most active drug ingredients and excipients, so incompatibilities are extremely rare. Adsorption of active components is possible, however the amounts are typically low and the process is reversible.
A summary of relevant toxicological data for Colloidal silicon dioxide is provided in the table below:
| Test | Result |
| Acute oral toxicity, Rat | LD50 > 3300 mg/kg |
| Acute inhalative toxicity, Rat | LCO: 0.139 mg/L/4hr (maximal attainable concentration) |
| Acute toxicity, Rabbit | LD50 > 5000 mg/kg |
| Eye Irritation, Rabbit | Non-irritating |
| Sin Irritation, Rabbit | Non-irritating |
| Mutagenicity (Ames test) | Negative |
The European Union’s Scientific Committee on Food (SCF) and the Joint FAO/WHO Expert Committee on Food Additives assessed the safety of different silica products and established a group acceptable daily intake (ADI) for silicon dioxide as “not specified”.
The highest amount of colloidal silicon dioxide (hydrophilic) approved so far per dosage form can be found in the FDA’s Inactive Ingredients Guide Database: http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm
With respect to public risks, the most likely sources of exposure to Colloidal silicon dioxide are through dermal contact or ingestion of natural or synthetic forms of silica, and less likely via inhalation. Occupational exposure, however, is more likely to arise via inhalation and skin contact.
Colloidal silicon dioxide does not pose any significant health effects to workers or end-users. The absence of adverse health effects is best summarized in the recommendations of the OECD SIDS Initial Assessment Report (OECD, 2004) which states Colloidal silicon dioxide is “currently of low priority for further work” due to the low exposure potential for humans. This is further supported by the available worker exposure data that continues to show there is no evidence of health hazards to workers from the manufacture and use of SAS products.
The long history of manufacture and use, coupled with data regarding the size distribution of the material, substantiates the widespread belief that colloidal silicon dioxide has a low potential for adverse health effects.
A debate on whether Colloidal silica particles fall under the definition of “nanomaterials” has been ongoing for some time now, and a resolution does not appear to be imminent. According to the definition of ISO TS 80004-1, Colloidal silicon dioxide (and other synthetic amorphous silicas) are nanostructured. On the other hand, the European Cosmetic Regulation (EC 1223/2009) defines “Nanomaterial” as an insoluble or bio-persistent and intentionally manufactured material having one or more external dimensions or an internal structure, on the scale from 1 to 100 nm, rules it out.
In numerous studies, it has been shown that in the bulk phase (normal existence), the primary particles in Colloidal silica do not exist as individual/discrete nanoparticles but are fused together to form aggregates, which subsequently form agglomerates. Therefore, the mean diameter of the resulting synthetic amorphous silica particles, as used, is typically in the micron range and well above 100 nm.
Colloidal silicon dioxide is generally chemically inactive. Provided it is stored appropriately and as recommended, the material does not undergo any significant chemical changes, even after several decades. The only caveat is that the material must not come into contact with hydrofluoric acid or strong alkalis, as these chemical substances chemically react with silicon dioxide.
Colloidal silicon dioxide has a low risk of aging or decomposition. The material can, in principle, be considered suitable for long-term storage and use when appropriately stored and handled. However, manufacturers recommend usage within two years of production for the following reasons:
When handling colloidal silicon dioxide, dust formation cannot be fully prevented, thus dust extraction procedures are recommended. A dust mask with particle filtering capability should be worn if high concentrations of dust are generated in the work environment.
When silica is being handled, electrostatic charging can occasionally occur, for example when the silica sack is being emptied. To avert any risk during handling, measures should therefore be taken to prevent electrostatic charging.
Spilled material should be collected without any dust being formed and placed in a suitable container.
Colloidal silicon dioxide is a synthetic substance made from two materials that are abundant in nature, namely silicon and oxygen. Analogues of Colloidal silicon dioxide occur in nature, and can be found in rocks, plants and animals. It is a versatile material that is used in plastics to improve their wear resistance, and has been used to replace microplastics. However, the commercial production of Colloidal silicon dioxide uses copious amounts of energy across the entire value chain. Surprisingly, when the sustainability of Colloidal silicon dioxide was assessed by the Excipients Forum, it achieved a score of 43 out of 100 on their Sustainable Chemistry™ scheme.
[1] Evonik Industries, AEROSIL(R) Pharma Colloidal silicon dioxide, Technical information.
[2] Evonik Industries, SIPERNAT and AEROSIL – an essential in industrial powder technology, Technical information.
[3] World Health Organization & Food and Agriculture Organization of the United Nations. (1991). Evaluation of certain food additives and contaminants : thirty-seventh report of the Joint FAO/WHO Expert Committee on Food Additives [meeting held in Geneva from 5 to 14 June 1990], (1991).
[5] G.K. Bolhuis, N. Anthony Armstrong, Excipients for direct compaction—an update, Pharmaceutical Development and Technology, 11 (2006) 111-124.
[6] L.C. Becker, W.F. Bergfeld, D.V. Belsito, R.A. Hill, C.D. Klaassen, D. Liebler, J.G. Marks, R.C. Shank, T.J. Slaga, P.W. Snyder, F.A. Andersen, Safety Assessment of Silylates and Surface-Modified Siloxysilicates, international Journal of Toxicology, 32 (2013).
[7] D. Majerová, L. Kulaviak, M. Růžička, F. Štěpánek, P. Zámostný, Effect of colloidal silica on rheological properties of common pharmaceutical excipients, European Journal of Pharmaceutics and Biopharmaceutics, 106 (2016) 2-8.
[8] Sccs, P.H.M. Hoet, Opinion of the Scientific Committee on Consumer Safety (SCCS) – Revision of the opinion on the safety of the use of Silica, Hydrated Silica, and Silica Surface Modified with Alkyl Silylates (nano form) in cosmetic products, regulatory toxicology and pharmacology, 74 (2016) 79-80.
[9] U.S.P. Convention, Food Chemicals Codex (11th Edition), (2018).
[11] Influence of mixing time, particle size and colloidal silica on the surface coverage and lubrication of magnesium stearate. Johansson, M. E., Nicklasson, M. Rubenstein, M. H. Ed.,Pharmaceutical Technology, Volume I, John Wiley & Sons, England, 1987
[12] Jonat, S., Hasenzahl, S., Gray, A., Schmidt, P. C. Influence of compacted hydrophobic and hydrophilic colloidal silicon dioxide on tableting properties of pharmaceutical excipients. Drug Development and Industrial Pharmacy 31 (2005) 687-696.
[13] Dal Zotto, M., Realdon, N., Ragazzi, E., Dalla Fini, G., Effects of two different kinds of silicon dioxide on release of drugs from suppositories: benzydamine hydrochloride. Farmaco 46 (5) (1991) 699-711.
The term ‘Silica’ (chemically silicon dioxide, SiO2) is applied and misapplied to many different materials that contain silica and oxygen, including free silica (SiO2) and silicates (which contain other elements). It occurs naturally, in several forms, the most obvious being sand.
Silica can also be obtained synthetically. Synthetic Amorphous Silica (SAS) exhibits a randomized internal structure (amorphous), and is distinct from natural or other synthetic crystalline silica types.
Silica, independent of its form and method of preparation (including by-products), is found under CAS Nr. 7631-86-9. However, as the different polymorphs of silica differ in their hazards to human health, it is essential, to distinguish carefully between crystalline silica and synthetic amorphous silica (crystalline-free). The situation can be complicated as natural forms of amorphous silica, unlike synthetic versions, often contain crystalline impurities (up to 65 % cristobalite in the case of calcinations).
An overview of silica types and CAS numbers is shown below:
Synthetic Amorphous Silica (SAS), EINECS No. 231-545-4, been produced and marketed for almost a century. SAS is used in a multitude of industrial applications. In addition, it is approved for use in food, cosmetics and pharmaceutical applications. All historic physicochemical and toxicology data remain valid for SAS manufactured today.
SAS is highly pure, crystalline-free, silicon dioxide, SiO2, which may be produced as pyrogenic (also called fumed) silica, precipitated silica and silica gel. SAS products are marketed as dry white powders or dispersions. SAS may sometimes be surface-treated to render it hydrophobic but these specialised products are produced at relatively low tonnage levels compared to the untreated material.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.
[bsa_pro_ad_space id=5]
Colloidal silicon dioxide is amorphous silica (oxide of silicon) prepared synthetically by the vapour-phase hydrolysis of a silicon compound. Colloidal silicon dioxide has the chemical formula SiO2 but is distinct from other types of silica, such as amorphous or crystalline silica, that exist naturally or otherwise such as silica gel or precipitated silica. Colloidal silicon dioxide is supplied as a white or almost white, light, fluffy, and extremely fine powder.
Pharmacopoeia Conformity: USP-NF; Ph. Eur; JP; IP; FCC
Synonyms & Trade Names: Silica Colloidal Anhydrous; Pyrogenic Silica; Fumed Silica; Colloidal Anhydrous Silicon Dioxide; Light Anhydrous Silicic Acid; Synthetic Amorphous Silica; SAS; Fumed Silicon Dioxide; Silicic Anhydride; Silicon Dioxide Colloidal; Hydrophilic Silica; E551; AEROSIL®; Cab-O-Sil®; Wacker HDK; AEROPERL® 300
Uses & Applications: Glidant; Anticaking Agent; Emulsion Stabilizer; Suspending Agent; Thermal Stabilizer; Viscosity-Increasing Agent; Desiccant, and Solubility-Enhancer

Colloidal silicon dioxide Anhydrous is a highly pure, very fine amorphous silicon dioxide having primary particles in the nano-scale range. It is obtained through chemical synthesis using a suitable silicon compound precursor, such as silicon tetrachloride, typically at temperatures >2000 oC. It is for this reason that it is also referred to as “fumed” or “pyrogenic” amorphous silicon dioxide. It is distinct from other types of silicon dioxide, whether natural or synthetic, amorphous or crystalline. In different pharmacopoeia, Anhydrous colloidal silicon dioxide is described as a fine, white, amorphous powder consisting of sub-microscopic particles (about 15 nm). It contains between 99.0 – 105 % of SiO2. It is insoluble in water, organic solvents and mineral acids.
Silicon and oxygen are the two most abundant elements in the earth’s crust. In nature, silicon almost always exists in combination with oxygen, either as free silica (SiO2), in conjunction with other elements (for instance, in silicates, which are the main minerals in rocks and soil), or as combined silica (SiO3). The different silicon compounds have substantially different chemical properties, applications, and hazards.
Free silica (Silicon dioxide) is a hard, low-reactivity, colourless substance that occurs naturally in rocks and minerals or can also be industrially produced in the form of synthetic amorphous silica. All forms of Silica, whether natural, synthetic, crystalline, cryptocrystalline or amorphous, are assigned a single CAS Registry number [7631-86-9]. For convenience, the different forms of Silica can be divided into three main groups as shown in the chart below:

At the fundamental level of Silica’s chemical properties is the Silica tetrahedron (SiO4), which consists of a central Silicon cation covalently bonded to four oxygen atoms, arranged in the shape of a tetrahedron. Silica tetrahedra may be linked and arranged in a variety of ways, from simple to complex three-dimensional frameworks. Crystalline forms of Silica exhibit a highly ordered crystal lattice, determined by the ordered arrangement of the Silica tetrahedra. Amorphous forms, on the other hand, have random, disordered lattices. The orientation of the bonds is random, and there is no long-range periodicity.
To differentiate between the different silica analogues, new CAS Registry numbers have been assigned. These are shown in the chart below:
![]()
Synthetic amorphous silicon dioxides are further divided into three main types, namely:
Even though they share the same chemical structure and synthetic origin, Synthetic silicas exhibit different properties, as briefly reviewed below:
Pyrogenic silicon dioxide is produced using a high-temperature process in which silicon tetrachloride is vaporised in an oxygen-hydrogen atmosphere according to the following chemical reaction:
SiCl4 + 2H2 + O2 -> SiO2 + 4HCl
The raw materials used in the silica production process are all inorganic and very pure. As a result, the synthesis produces only hydrochloric acid (which is easily removed) and silica in very high states of purity (typically > 99.9%). Silicon dioxide produced pyrogenically exists in the form of chain-like, branched aggregates, giving rise to a fluffy, light powder. (The term “fume” alludes to the method of manufacture, which involves the use of a flame.) Varying processing conditions allows the production of silica products with different specific surface areas, typically between 50 and 400 m2/g.
The pyrogenic method for producing Silica was invented in 1941 by Harry Klopfer, a scientist at Degussa (now part of Evonik AG). This method is what is still used by Evonik (for the production of pyrogenic silica marketed under the AEROSIL® brand name) and Cabot Corporation (for the Cab-O-sil® fumed silica brand). Note that these silica grades can be used in their native (unprocessed) state. They can also be further processed (for example, spray drying, granulation, or surface chemical modification) to turn them into other technical silica grades.
Precipitated silicon dioxide is silica produced in an aqueous solution at temperatures >60 °C. In this process, sodium silicate (waterglass) undergoes controlled neutralisation with either concentrated sulphuric acid or hydrochloric acid. The Silica precipitates out as a slurry of (hydrated) silica, which is washed and filtered to remove by-products. It is then dried in hot air and milled or passed through a classifier.
Precipitated silicon dioxide has been known since the mid 17th century. It was not until the 1920s that its practical uses and industrial production were fully established. Currently, Precipitated silica is produced in volumes that are up to x10 greater than for Pyrogen silica. The method and conditions have been fine-tuned and now permit production of many types of synthetic silica that are structurally and characteristically different, including exhibiting internal pore volume/specific surface area, larger particle sizes, and water content.
3). Surface-Modified Silicon Dioxide
The Silica grades described thus far are available for use as in their native or unmodified state. These materials have freely accessible silanol groups (Si-OH) on the surfaces of Silica particles, rendering them hydrophilic. Frequently, it is desirable to have hydrophobic silica, i.e a product that repels water. Hydrophobicity can be achieved through a post-synthesis step in which the silanol groups are reacted with organic groups. The added organic groups are tightly bound to the surface (via covalent bonds) and are only broken via thermal decomposition.

Pharmaceutical-approved hydrophobic silica is produced by reacting hydrophilic silicon dioxide with dimethylchlorosilane immediately after the production of Silica particles in the hydrogen flame chamber. This process is also conducted at high temperatures and allows dimethysilyl groups to be bound irreversibly onto the surface of the silica via siloxane bonds. This produces a material that, while appearing identical to the precursor Silica, is very hydrophobic, repels water and does not absorb moisture from the environment.
“Colloidal” is used in reference to both pyrogenic and precipitated silica. It may be confused with Silica colloids, which are also obtained via the wet chemical route. Note that the International Union of Pure and Applied Chemistry (IUPAC) defines colloids as systems (dispersions) in which particles of colloidal size (1 nm–1000 nm) of any nature (solid, liquid, or gas) are dispersed in a continuous phase of different composition or state.
Thus, in the strictest sense, the term ‘colloidal silica’ applies to stable dispersions (or sols) consisting of discrete particles of amorphous silica having sizes of between 5 and 100 nm. These colloidal silicas are commercially available in the form of sols or dried powders (e.g., xerogels, dry precipitates, aerogels, or calcinated coarcervates). In a broad sense, however, many other forms of silica (other than wet or dry silica sols above) are colloidal on the grounds that they are composed of particles in a colloidal state of subdivision (1-1000 nm).
It is also worth noting that the silica particles and aggregates are self-supporting and stabilised dispersions of silica particles in a continuous air phase and are unaffected by gravitational forces. Finally, fumed silica is commonly referred to as ‘colloidal’ because the silica powders are made by condensing a silica precursor from a vapour phase. In this sense, fumed silica particles are dispersed in a gaseous phase during production process.
The SiO2 molecules in synthetic silicon dioxide do not exist in isolation. While the primary structure is the tetrahedron, consisting of one silicon atom bonded to four oxygen atoms, tetrahedrons arrange into networks. During the synthesis process, minute droplets of SiO2 initially aggregate into so-called nuclides, which combine stochastically into even larger particles, facilitated by weak physical interactions such as van der Waal’s forces.
Another important property of synthetic silica is its specific surface area. Fumed silicon dioxide, in particular, has only one surface, which is external and little or non-existent internal pore volume. Precipitated silica, on the other hand, is mesoporous and exhibits an internal surface. Generally, the higher the specific surface area, the greater the degree of agglomeration. It is these aggregates that partly contribute to the unique functionalities of amorphous silicon dioxide.

| Chemical Name | Silicon dioxide, chemically synthesised |
| CAS Registration Number | [112945-52-5] |
| Empirical Formula | SiO2 |
| Molecular weight | 60.08 |
| EC Number | 231-545-4 |
| UNII Code (FDA) | ETJ7Z6XBU4 |
Colloidal silicon dioxide is approved for use in pharmaceutical products as an excipient (oral, topical products and topical sprays but not for inhalation). It is listed in all the major pharmacopoeia, including the USP-NF, Ph.Eur, I.P, B.P, and J.P.E. A specification for Colloidal silicon dioxide is included in the Food Chemicals Codex (FCC). It has been used in pharmaceuticals, food products, and cosmetics for over 50 years.
Colloidal silicon dioxide is currently approved by the FDA, is GRAS listed and included in the FDA Inactive Ingredients Database. Both the European Union and the FDA have authorised the use of Colloidal silicon dioxide as a food additive and for food contact materials. In Europe, Colloidal silica is designated E551. The following regulatory schedules apply to colloidal silicon dioxide:
| Jurisdiction | Framework | Record | Comment |
| European Union | European Pharmacopoeia | Monograph – ‘Silica, Colloidal Anhydrous’ | |
| Food Additive | E551, as Silicon Dioxide | ||
| Animal Nutrition | E551a and E551b | ||
| United States of America | United States Pharmacopoeia | Monograph – ‘Colloidal Silicon Dioxide’ | |
| Food Additive and Contact Material | FCC specification – ‘Silicon Dioxide’ | Limits apply | |
| Animal Nutrition | 21 CFR.133 & 160 | Limits apply | |
| Japan | Japanese Pharmacopoeia | Monograph – ‘Colloidal Silicon Dioxide’ | |
| Animal Nutrition | Food Safety & Quality regulation |
Several grades of colloidal silicon dioxide are widely available, depending mainly on the method of production, and any subsequent processing changes that the material is subjected to. These changes, while not necessarily affecting the silica content, density, refractive index, colour, or amorphous form, do impact three key parameters, i.e:
As a result, different commercial grades of colloidal silicon dioxide, such as AEROSIL® (Evonik), Cab-O-Sil (Cabot Corporation) and Wacker HDK (Wacker-Chemie GmbH) may differ in these parameters. The table below provides a synopsis of the main physicochemical properties of importance.
| Physical form | Solid, powder |
| Appearance | White |
| Flowability | Fluffy, poorly flowing or free-flowing powder |
| Hygroscopicity | Colloidal silica is hygroscopic. Moisture content is dependent on grade and storage conditions |
| pH value | pH 3.5 – 5.5 (4% w/v aqueous dispersion) |
| Poured Density | 0.03 – 0.04 g/ml |
| Tapped Density | Dependent on grade |
| Melting point | 16000C |
| Particle size distribution | Primary particle size are in nano range (5 – 20 nm). However, most Silica exists in the form of loose agglomerates of 10 to ≥100µm. |
| Refractive index | 1.46 |
| Solubility | Practically insoluble in water, propylene glycol, glycerol, organic solvents, mineral oil, and most acids. Soluble in hot solutions of alkali hydroxide. |
| Relative density | 2.2 |
| BET Specific surface area | 50 – 600 m2/g depending on grade and supplier |
| USP-NF | Ph.Eur | J.P | I.P | |
| Name | Colloidal Silicon Dioxide | Silica, Colloidal Anhydrous | Light Anhydrous Silicic Acid | Silica, Colloidal Anhydrous |
| Authorised Uses | Excipient | Excipient | Excipient | Excipient |
| Definition | specified | specified | specified | specified |
| Characters | n/a | specified | n/a | specified |
| Identification | specified | specified | specified | specified |
| pH | 3.5-5.5 | 3.5-5.5 | n/a | 3.5-5.5 |
| Chlorides | na | ≤250 ppm | ≤0.011 % | ≤250 ppm |
| Aluminium | specified | n/a | n/a | n/a |
| Iron | n/a | n/a | ≤500 ppm | n/a |
| Calcium | specified | n/a | n/a | n/a |
| Arsenic | ≤ 8 ppm | n/a | ≤ 5 ppm | ≤8 ppm |
| Heavy Metals | na | ≤ 25 ppm | ≤ 40 ppm | ≤ 25 ppm |
| Loss on Ignition (%) | ≤ 2.0% | ≤ 5.0% | 12.0 % | ≤ 5.0 % |
| Loss on Drying (%) | ≤ 2.5% | n/a | 7.0 | n/a |
| Volume Test | n/a | n/a | n/a | ≤ 70 ml |
| Assay (%) | 99.0-100.5 | 99.0-100.5 | ≥98.0 | 99.0-100.5 |
| Labelling | Specified | n/a | n/a | n/a |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Colloidal silicon dioxide is one of the most widely used pharmaceutical excipients today. It is used in solid and liquid dosage forms, food products, and cosmetics and has been in use for over 60 years. Its usefulness stems from three key attributes:
Colloidal silicon dioxide is used as a carrier for liquids and semi-liquids or as free-flow agent in powdered products (pharmaceuticals, cosmetics, salts, and foods), particularly for substances that attract water molecules (hygroscopic) or cake, so that they flow better.
It also provides pastes and ointments with the desired consistency and prevents separation of the various formulation ingredients, making it a highly versatile material with multiple pharmaceutical and cosmetic applications. This versatility stems from its high absorptive capacity, ability to improve flow, and low dust content, as outlined in the table below:
| Application | Important Properties |
| Absorbent/desiccant | Porosity |
| Matting, gelling and structuring effects | Aggregate particle size |
| Toothpaste | Aggregate particle size |
| Elastomer reinforcement | Aggregate particle size |
| Free-flow, anti-caking & glidant | Porosity, aggregate particle size |
| Rheology control | Surface chemistry |
When applied to pharmaceutical products, the above-listed Silica attributes provide the following functionalities:
Thus, Colloidal silicon dioxide functions, in both solid and liquid dosage forms, as a:
In tablets, capsules, and granules, Colloidal silicon dioxide is popularly used as a glidant and anti-adherent, where it allows powder formulations to efficiently flow on high-speed tablet presses. The available grades and their uses are outlined in the table below:
| Product | Properties | Benefits |
| AEROSIL® 200 Pharma (Evonik)
CAB-O_SIL® M5 PD (Cabot) HDK® N20 Pharma (Wacker) |
Hydrophilic silica. 175-225 m2/g (BET Surface area). Untreated surface. | Traditional glidant for solid dosage forms. Suitable for formulating soft powder formulations. Thickener for oils |
| AEROSIL® 200 VV Pharma (Evonik)
HDK® N20P Pharma |
Hydrophilic, densified silica. 175-225 m2/g (BET Surface area). Untreated surface | Glidant and thickener applications but with reduced dusting and less volume requirements |
| AEROSIL® 300 Pharma (Evonik) | Hydrophilic silica. 270-330 m2/g (BET Surface area). Untreated | Ideal glidant for hygroscopic powders that exhibit particle-particle cohesiveness via liquid bridge formation |
Glidants permit maximization of throughput on high-speed machinery while also ensuring products meet quality and regulatory requirements for dose and content uniformity.
A key advantage of Colloidal silicon dioxide is that only a small quantity is needed to improve the flowability and compressibility of a powdered formulation, which yields strong tablets and higher content uniformity. For most formulations, standard hydrophilic Colloidal silicon dioxide grades are sufficient due to their ability to efficiently adsorb moisture and keep powders dry and free flowing (typical usage levels is 0.1-0.5%).
How Glidants Work
Several forces are at play when it comes to particle-particle interactions, and, thus, powder cohesiveness and flow. In general, these forces include but are not limited to van der Waals forces, electrostatic forces, liquid bridges, and physical entanglements. The smaller the particles, the greater the interactions, and thus, the more cohesive a powder is. This is the reason micronised powders do not flow well.
Colloidal silicon dioxide improves powder flow by counteracting the forces behind particle cohesion. This is achieved by adhering to the surface, increasing the distance, and reducing attractive forces (van der Waals forces and electrostatic attraction decrease with increasing distance between the particles).
In addition, the hydrophilic nature of Colloidal silicon dioxide enables it to attract and preferentially bind moisture, helping to eliminate liquid bridges between solid particles that hinder powder flow.
Finally, aggregates of Colloidal silicon dioxide occupy and fill voids, which eliminates irregularities on the particle surfaces, reducing particle-particle entanglements.
How to Use Colloidal Silica As a Glidant
Colloidal silicon dioxide is used in tablet coating processes due to its ability to improve throughput and economics. In standard coating processes involving powder layering, Colloidal silicon dioxide is used to facilitate powder build-up in the coating while also helping maintain pigments in suspension.
In acquiring good flowability (lubricity), the build-up powder distributes more evenly on the cores and dries much faster, which shortens the entire coating process considerably. Meanwhile, much stronger coats are obtained since the cores spend relatively shorter time periods in the coater. The high moisture binding capacity of Colloidal silicon dioxide also protects cores from over wetting.
Whether powder build-up is used, or only highly concentrated coating suspensions are used, Colloidal silicon dioxide is still useful for stabilising pigment suspension and improving a coating’s smoothness. In this regard, the recommended levels of Colloidal silicon dioxide for use in coating build-up are 10 to 15% w/w, while the levels for aiding pigment suspension are 0.5 to 2.0% w/w.
Colloidal silicon dioxide grades find utility in liquid formulations on account of their viscosity-modifying properties as well as their ability to prevent particle settling in disperse (suspensions) systems. Both effects are based on the ability of dispersed Colloidal silica particles to form a network of aggregates via hydrogen bridge linkage and/or van der Waals attractive forces in liquid media.
Thus, when the fumed silica is dispersed in a liquid, silanol groups interact with each other either directly or indirectly via the molecules in the liquid. This affinity is attributed to hydrogen bonding, and results in a reversible, three-dimensional lattice structure that manifests macroscopically as thickening.
When the system is mechanically sheared, such as through shaking, the structure is broken down, rendering the system more fluid as the viscosity drops. At rest, however, the lattice rebuilds, and the viscosity returns to what it was before, a phenomenon known as thixotropy.
Several nonpolar liquids, such as vegetable oils, liquid paraffin and isopropyl myristate, can be converted into spreadable gels with Colloidal silicon dioxide. Provided the liquid has a refractive index closer to that of the silicon dioxide (i.e., 1.48), generally transparent gels will be obtained.
A key feature of gels obtained with colloidal silica that makes them different from other types of gels (for instance, those obtained with hydrocolloid gums) is that their viscosities show little dependence on temperature. This makes them well-suited for formulations whose storage and temperature stabilities are critical.
Generally, the viscosity of obtained gels depends on the amount of silica added; that is, the more is added, the thicker the gel will be. Any standard silica grade can be used, although grades with much larger specific surface areas, such as Evonik’s AEROSIL® 300 Pharma are the most recommended. Densified grades are not used to thicken oils because they form larger, more stable agglomerates that are difficult to disperse.
How to Use Colloidal Silica to Thicken Oils
Colloidal silicon dioxide is an important ingredient in the formulation and production of suppositories. It allows formulation ingredients to be uniformly distributed throughout the suppository base.
In addition, it increases the softening point of the suppository base without changing its melting point, an important property for improving stability in warm climates. The consistency and mechanical stability of the finished suppository are also improved.
In the unlikely event that an active ingredient inadvertently reduces the melting point of the suppository base (especially solution suppositories), silica can be added to prevent this phenomenon. The recommended procedure is to initially “triturate” the offending ingredient with the colloidal silicon dioxide before adding it to the product.
Colloidal silicon dioxide can be used in membrane-controlled and matrix-controlled transdermal drug delivery systems as a gel former or to increase storage and thermal stability. It can also be used as a carrier or adsorbent for active ingredients and to improve incorporation or release characteristics.
The recommended concentrations of colloidal silica in transdermal drug delivery systems are generally between 1 and 5 % w/w. The silica should be dispersed in the medium that contains the active ingredient, polymer (adhesive) and/or other excipients.
Colloidal silicon dioxide is an effective excipient for stabilizing dispersions of solids in liquids and preventing the formation of hard sediments in liquid suspensions and aerosol formulations intended for topical (rather than inhalation) use.
Colloidal silicon dioxide allows suspended particles to remain in suspension long enough until they are deposited on the substrate, for instance, the skin, or in the case of coatings, the tablet core. The recommended usage levels are generally between 0.5 to 3.0% w/w.
Systemically acting drug products formulated as solid oral dosage forms necessarily require their active pharmaceutical ingredients (APIs) to dissolve in gastrointestinal juices before absorption into the bloodstream can occur. Therefore, APIs must first undergo dissolution in gastric juices and successfully permeate the lipid membrane of the intestinal wall. APIs that exhibit high solubility and high permeability generally have high bioavailability.
The importance of drug dissolution and permeability has been previously described by the Biopharmaceutics Classification System (BCS), originally proposed by Amidon, which groups APIs into four different classes according to their permeability and solubility in aqueous media, namely, BCS I, II, III and IV. Pharmaceutical scientists have used many different technologies to improve the solubility of products exhibiting poor solubility and/or permeability, including API modification, lipid formulations, solid dispersions, and speciality techniques (liposomes, prodrugs, etc).
Speciality granulated silica grades consisting of spherical particles in the range of 10–100 μm that feature a high internal surface (mesoporous) and high density can be used in the above-mentioned formulation strategies aimed at enhancing solubility and dissolution. An example of such mesoporous Colloidal silicon dioxide is AEROPERL® 300 Pharma (Evonik AG). AEROPERL® 300 Pharma, which like standard Colloidal silicon dioxide, is tested against the high-quality requirements of the “Silica Colloidal Anhydrous” (Ph. Eur) and “Colloidal Silicon Dioxide” (USP/NF) monographs.
Mesoporous Silica can be used to improve API solubility in a number of ways, as outlined below:
Moisture Activated Dry Granulation (MADG) is an alternative granulation technique that uses very small amounts of water (2-4% w/w) to initiate granule formation. Compared with traditional wet granulation, MADG does not involve the use of heat to dry granules and involves fewer process steps.
The process steps involved in MADG are shown below:
MADG was developed by Bristol Myers Squibb in the 1980s to address the challenges associated with wet granulation, including end-point determination, drying, milling, and moisture-instigated instability from hydrolysis.
A grade of mesoporous Colloidal silicon dioxide featuring a high specific surface area can be used as a moisture desiccant in MADG. The silica, together with other excipients, helps absorb and distribute the moisture, which yields uniform, free-flowing, and compactible granules.
Note that the aim of the MADG process is not to make large granules, but rather to agglomerate fines and bind the drug with excipients into freely flowing, compressible granules that can be tabletted without further processing.
As a result, MADG offers multiple advantages, including reduced energy costs, fewer processing steps, and reduced investment in machinery.
Colloidal silicon dioxide is a purely inorganic substance. The production process does not involve plant or animal based raw materials, nor does it involve organic solvents. It has been produced and utilised in medical and food products for over six decades and has been extensively studied by health authorities and many other scientific organisations.
For a long time, Colloidal silicon dioxide was incorrectly associated with crystalline forms of silica since the two materials shared the same name and chemical formula. Crystalline silicas have been shown to be responsible for many of the deleterious effects, such as silicosis. On the other hand, silicon dioxide grades that are both synthetic and amorphous in structure do not share the biologic hazards. They do not cause silicosis. However, they should not be used for drug delivery by inhalation or injection because of the risks of tissue reactions or the formation of granulomas.
Evidence obtained from the many studies done over several decades has led authorities to conclude that colloidal silicon dioxide has minimal adverse health effects from exposure, and is not harmful when administered orally or dermally. It is not irritating to skin and eyes and passes through the gastrointestinal tract without being assimilated in detectable amounts.
The European Union’s Scientific Committee on Food (SCF) concluded that silicon dioxide did not raise concerns with respect to genotoxicity. However, it should not be administered parenterally, because untoward tissue reactions or the formation of granulomas could occur.
Finally, colloidal silicon dioxide is inert toward most active drug ingredients and excipients, so incompatibilities are extremely rare. Adsorption of active components is possible, however the amounts are typically low and the process is reversible.
A summary of relevant toxicological data for Colloidal silicon dioxide is provided in the table below:
| Test | Result |
| Acute oral toxicity, Rat | LD50 > 3300 mg/kg |
| Acute inhalative toxicity, Rat | LCO: 0.139 mg/L/4hr (maximal attainable concentration) |
| Acute toxicity, Rabbit | LD50 > 5000 mg/kg |
| Eye Irritation, Rabbit | Non-irritating |
| Sin Irritation, Rabbit | Non-irritating |
| Mutagenicity (Ames test) | Negative |
The European Union’s Scientific Committee on Food (SCF) and the Joint FAO/WHO Expert Committee on Food Additives assessed the safety of different silica products and established a group acceptable daily intake (ADI) for silicon dioxide as “not specified”.
The highest amount of colloidal silicon dioxide (hydrophilic) approved so far per dosage form can be found in the FDA’s Inactive Ingredients Guide Database: http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm
With respect to public risks, the most likely sources of exposure to Colloidal silicon dioxide are through dermal contact or ingestion of natural or synthetic forms of silica, and less likely via inhalation. Occupational exposure, however, is more likely to arise via inhalation and skin contact.
Colloidal silicon dioxide does not pose any significant health effects to workers or end-users. The absence of adverse health effects is best summarized in the recommendations of the OECD SIDS Initial Assessment Report (OECD, 2004) which states Colloidal silicon dioxide is “currently of low priority for further work” due to the low exposure potential for humans. This is further supported by the available worker exposure data that continues to show there is no evidence of health hazards to workers from the manufacture and use of SAS products.
The long history of manufacture and use, coupled with data regarding the size distribution of the material, substantiates the widespread belief that colloidal silicon dioxide has a low potential for adverse health effects.
A debate on whether Colloidal silica particles fall under the definition of “nanomaterials” has been ongoing for some time now, and a resolution does not appear to be imminent. According to the definition of ISO TS 80004-1, Colloidal silicon dioxide (and other synthetic amorphous silicas) are nanostructured. On the other hand, the European Cosmetic Regulation (EC 1223/2009) defines “Nanomaterial” as an insoluble or bio-persistent and intentionally manufactured material having one or more external dimensions or an internal structure, on the scale from 1 to 100 nm, rules it out.
In numerous studies, it has been shown that in the bulk phase (normal existence), the primary particles in Colloidal silica do not exist as individual/discrete nanoparticles but are fused together to form aggregates, which subsequently form agglomerates. Therefore, the mean diameter of the resulting synthetic amorphous silica particles, as used, is typically in the micron range and well above 100 nm.
Colloidal silicon dioxide is generally chemically inactive. Provided it is stored appropriately and as recommended, the material does not undergo any significant chemical changes, even after several decades. The only caveat is that the material must not come into contact with hydrofluoric acid or strong alkalis, as these chemical substances chemically react with silicon dioxide.
Colloidal silicon dioxide has a low risk of aging or decomposition. The material can, in principle, be considered suitable for long-term storage and use when appropriately stored and handled. However, manufacturers recommend usage within two years of production for the following reasons:
When handling colloidal silicon dioxide, dust formation cannot be fully prevented, thus dust extraction procedures are recommended. A dust mask with particle filtering capability should be worn if high concentrations of dust are generated in the work environment.
When silica is being handled, electrostatic charging can occasionally occur, for example when the silica sack is being emptied. To avert any risk during handling, measures should therefore be taken to prevent electrostatic charging.
Spilled material should be collected without any dust being formed and placed in a suitable container.
Colloidal silicon dioxide is a synthetic substance made from two materials that are abundant in nature, namely silicon and oxygen. Analogues of Colloidal silicon dioxide occur in nature, and can be found in rocks, plants and animals. It is a versatile material that is used in plastics to improve their wear resistance, and has been used to replace microplastics. However, the commercial production of Colloidal silicon dioxide uses copious amounts of energy across the entire value chain. Surprisingly, when the sustainability of Colloidal silicon dioxide was assessed by the Excipients Forum, it achieved a score of 43 out of 100 on their Sustainable Chemistry™ scheme.
[1] Evonik Industries, AEROSIL(R) Pharma Colloidal silicon dioxide, Technical information.
[2] Evonik Industries, SIPERNAT and AEROSIL – an essential in industrial powder technology, Technical information.
[3] World Health Organization & Food and Agriculture Organization of the United Nations. (1991). Evaluation of certain food additives and contaminants : thirty-seventh report of the Joint FAO/WHO Expert Committee on Food Additives [meeting held in Geneva from 5 to 14 June 1990], (1991).
[5] G.K. Bolhuis, N. Anthony Armstrong, Excipients for direct compaction—an update, Pharmaceutical Development and Technology, 11 (2006) 111-124.
[6] L.C. Becker, W.F. Bergfeld, D.V. Belsito, R.A. Hill, C.D. Klaassen, D. Liebler, J.G. Marks, R.C. Shank, T.J. Slaga, P.W. Snyder, F.A. Andersen, Safety Assessment of Silylates and Surface-Modified Siloxysilicates, international Journal of Toxicology, 32 (2013).
[7] D. Majerová, L. Kulaviak, M. Růžička, F. Štěpánek, P. Zámostný, Effect of colloidal silica on rheological properties of common pharmaceutical excipients, European Journal of Pharmaceutics and Biopharmaceutics, 106 (2016) 2-8.
[8] Sccs, P.H.M. Hoet, Opinion of the Scientific Committee on Consumer Safety (SCCS) – Revision of the opinion on the safety of the use of Silica, Hydrated Silica, and Silica Surface Modified with Alkyl Silylates (nano form) in cosmetic products, regulatory toxicology and pharmacology, 74 (2016) 79-80.
[9] U.S.P. Convention, Food Chemicals Codex (11th Edition), (2018).
[11] Influence of mixing time, particle size and colloidal silica on the surface coverage and lubrication of magnesium stearate. Johansson, M. E., Nicklasson, M. Rubenstein, M. H. Ed.,Pharmaceutical Technology, Volume I, John Wiley & Sons, England, 1987
[12] Jonat, S., Hasenzahl, S., Gray, A., Schmidt, P. C. Influence of compacted hydrophobic and hydrophilic colloidal silicon dioxide on tableting properties of pharmaceutical excipients. Drug Development and Industrial Pharmacy 31 (2005) 687-696.
[13] Dal Zotto, M., Realdon, N., Ragazzi, E., Dalla Fini, G., Effects of two different kinds of silicon dioxide on release of drugs from suppositories: benzydamine hydrochloride. Farmaco 46 (5) (1991) 699-711.
The term ‘Silica’ (chemically silicon dioxide, SiO2) is applied and misapplied to many different materials that contain silica and oxygen, including free silica (SiO2) and silicates (which contain other elements). It occurs naturally, in several forms, the most obvious being sand.
Silica can also be obtained synthetically. Synthetic Amorphous Silica (SAS) exhibits a randomized internal structure (amorphous), and is distinct from natural or other synthetic crystalline silica types.
Silica, independent of its form and method of preparation (including by-products), is found under CAS Nr. 7631-86-9. However, as the different polymorphs of silica differ in their hazards to human health, it is essential, to distinguish carefully between crystalline silica and synthetic amorphous silica (crystalline-free). The situation can be complicated as natural forms of amorphous silica, unlike synthetic versions, often contain crystalline impurities (up to 65 % cristobalite in the case of calcinations).
An overview of silica types and CAS numbers is shown below:

Synthetic Amorphous Silica (SAS), EINECS No. 231-545-4, been produced and marketed for almost a century. SAS is used in a multitude of industrial applications. In addition, it is approved for use in food, cosmetics and pharmaceutical applications. All historic physicochemical and toxicology data remain valid for SAS manufactured today.
SAS is highly pure, crystalline-free, silicon dioxide, SiO2, which may be produced as pyrogenic (also called fumed) silica, precipitated silica and silica gel. SAS products are marketed as dry white powders or dispersions. SAS may sometimes be surface-treated to render it hydrophobic but these specialised products are produced at relatively low tonnage levels compared to the untreated material.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.