Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Sodium Starch Glycolate is a pharmaceutical excipient and superdisintegrant composed of the sodium salt of cross-linked carboxymethyl starch. It is widely recognised in the pharmaceutical industry for its adaptability and compatibility with many active pharmaceutical ingredients, and processing technologies. It is supplied as a white or almost white, free-flowing very hygroscopic powder.
Pharmacopoeia Compliance: USP-NF; Ph.Eur; IP, J.P; B.P; ChP
Synonyms & Trade Names: Sodium Stearyl Glycollate; Carboxyl Methyl Starch; SSG; E460; VIVASTAR®; EXPLOTAB®; PRIMOJEL®; EXPLOSOL®; GLYCOLYS®; TABLO®;
Uses & Applications: Tablet and Capsule Superdisintegrant
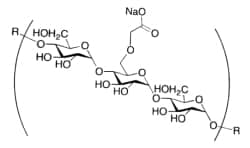
Sodium starch glycolate is a modified potato starch excipient (and corresponds to the chemical name, Sodium carboxymethyl starch). Chemical modification is achieved through controlled cross-linking of the starch polymer backbone, which is followed by the introduction of carboxymethyl sodium groups (on C2 of the glucose molecule). These changes (1) increase the hydrophilicity of Sodium starch glycolate, and (2) reduce its water solubility and gelling tendency, giving rise to the functionality of Sodium starch glycolate as a tablet superdisintegrant.
The Ph.Eur additionally describes Sodium starch glycolate by its appearance, noting that when examined under a microscope, Sodium starch glycolate consists of irregularly-shaped granules, which may be ovoid or pear-shaped, 30–100 µm in size, or rounded, 10–35 µm in size. The granules have an eccentric hilum and clearly visible concentric striations, as shown in this scanning electron micrograph below:

Sodium starch glycolate can be characterized either by the degree of substitution (DS), which varies between 0.23 and 0.32, or the degree of crosslinking in the polymer molecule. The molecular weight varies between 5 x l05 – 1 x 106 gram/mol. In the two main pharmacopoeia (Ph.Eur or USP-NF), several grades of Sodium starch glycolate are listed, as follows:
All the three types of Sodium starch glycolate (A, B, and C) are differentiated by their pH, sodium, and sodium chloride content. Only Type A and Type B are harmonized in the USP-NF and Ph.Eur monographs.
Since its introduction, Sodium starch glycolate has emerged as one of the most widely used disintegrants in oral solid dosage pharmaceuticals, both in capsule and tablet formulations. It is unique because its effectiveness is unaffected by the presence of hydrophobic excipients. Increasing the tablet compression pressure also has no effect on disintegration. These properties have helped make it a favourite within the pharmaceutical formulator community.
The mechanism by which Sodium starch glycolate achieves disintegration is wicking, rapid swelling and deaggregation of granules. Uptake of water and swelling are extremely rapid, meaning that most products in which Sodium Starch Glycolate is used will disintegrate fully within 3 minutes.
| Chemical Name | Starch carboxymethyl ether, sodium salt |
| CAS Registry Number | [9063-38-1] |
| Empirical Formula | (C6H10O5)n.(Na)x |
| Molecular Weight | 5 x l05 – 2 x 106 Daltons |
| EC/EINECS Number | 618-597-7 |
| UNII Code (FDA) | H8AV0SQX4D |
Sodium starch glycolate is an approved pharmaceutival excipient. It is currently approved for use in oral pharmaceutical products and is listed in all major pharmacopoeias, including the USP-NF, Ph.Eur, BP and IP. It is also listed in the JPE. Sodium starch glycolate is included in the FDA Inactive Ingredients Database.
| Physical state | Solid powder |
| Appearance | White powder |
| pH value | Not applicable |
| Poured (Bulk) Density | 0.65-0.85
0.756 g/cm3 (GLYCOLYS®), 0.81 g/cm3 (PRIMOJEL®), 0.67 g/cm3 (TABLO®) |
| Tapped Density | 0.945 g/cm for (GLYCOLYS®), 0.98 g/cm3 for (PRIMOJEL®), |
| True Density | 1.56 g/cm3 (PRIMOJEL®), 1.49 g/cm3 for TABLO® |
| Melting point | Chars at 200 0C before melting |
| Particle size distribution | D50 = 35 – 45 µm depending on grade. 100% ≤ 110 μm |
| Solubility | Insoluble in organic solvents. Yields a turbid suspension when dispersed in water |
| Specific surface area | Varies between grades. 0.23-0.25 m2/g (GLYCOLYS®), 0.185m2/g (PRIMOJEL®) |
| Swelling capacity | Up to 300 times its volume |
| Viscosity (dynamic) | ≤ 200 mPas (4% w/v aqueous dispersion) |
| USP-NF | Ph.Eur | B.P | |
| Name | Sodium starch glycolate | Sodium starch glycolate | Sodium starch glycolate |
| Authorised uses | Excipient | Excipient | Excipient |
| Definition | specified | specified | specified |
| Identification | specified | specified | specified |
| Characters | specified | specified | specified |
| Appearance of solution | specified | specified | specified |
| pH
Type A Type B Type C |
≤ 5.5 – 7.5 ≤ 3.0 – 5.0 ≤ 5.5 – 7.5 |
≤ 5.5 – 7.5 ≤ 3.0 – 5.0 ≤ 5.5 – 7.5 |
≤ 5.5 – 7.5 ≤ 3.0 – 5.0 ≤ 5.5 – 7.5 |
| Sodium glycolate
Type A Type B Type C |
≤ 2.0% ≤ 2.0% ≤ 2.0% |
≤ 2.0% ≤ 2.0% ≤ 2.0% |
≤ 2.0% ≤ 2.0% ≤ 2.0% |
| Heavy metals | ≤ 20 ppm | ≤ 20 ppm | ≤ 20 ppm |
| Iron | ≤ 20 ppm | ≤ 20 ppm | ≤ 20 ppm |
| Loss on drying
Type A Type B Type C |
≤ 10.0% ≤ 10.0% ≤ 7.0% |
≤ 10.0% ≤ 10.0% ≤ 7.0% |
≤ 10.0% ≤ 10.0% ≤ 7.0% |
| Microbial limits | specified | specified | specified |
| Sodium chloride
Type A Type B Type C |
≤ 7.0% ≤ 7.0% ≤ 1.0% |
≤ 7.0% ≤ 7.0% ≤ 1.0% |
≤ 7.0% ≤ 7.0% ≤ 1.0% |
| Assay (of Na)
Type A Type B Type C |
2.8 – 4.2% 2.0 – 3.4% 2.8 – 5.0% |
2.8 – 4.2% 2.0 – 3.4% 2.8 – 5.0% |
2.8 – 4.2% 2.0 – 3.4% 2.8 – 5.0% |
| Labelling | specified | n/a | n/a |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
The main (and only) function of Sodium starch glycolate in pharmaceutical products is as a tablet and capsule disintegrant. It is one of the most frequently used excipients in oral pharmaceuticals, accounting for 30% of market share of disintegrants. It is also suitable for use in wet granulation, direct compression, roller compaction, and extrusion-spheronisation. The typical concentrations in formulation are between 1% and 4 % for tablets, and 4–8% in capsules, with the optimum concentration being around 4%.
The mechanism by which Sodium starch glycolate achieves disintegration is wicking, rapid swelling, and deaggregation of granules. Uptake of water and swelling are extremely rapid, meaning that most products in which Sodium starch glycolate is used will disintegrate fully within 3 minutes, irrespective of the compression pressure applied.
Among superdisintegrants, Sodium starch glycolate is unique because its effectiveness is unaffected by the presence of hydrophobic excipients. Increasing the tablet compression pressure also has no effect on disintegration. These properties have helped make it one of the most popular superdisintegrants within the pharmaceutical formulator community.
Features and Benefits
| Feature | Type A | Type B | Type C |
| Identity by IR
Ph.Eur USP-NF JPE |
No Yes Yes |
No Yes Yes |
No No monograph No monograph |
| Assay (Na)
Ph.Eur USP-NF JPE |
2.8-4.2 2.8-4.2 2.8-4.2 |
2.0-3.4 2.0-3.4 2.0-3.4 |
2.8-5.0 No monograph No monograph |
| pH
Ph.Eur USP-NF JPE |
5.5-7.5 5.5-7.5 5.5-7.5 |
3.0-5.0 3.0-5.0 3.0-5.0 |
5.5-7.5 No monograph No monograph |
| Loss on Drying | Max 10% | Max 10% | Max 7% |
| Uses | Superdisintegrant in tablets & capsules offering rapid and high swelling.
Ideal for poorly soluble actives |
There are no rules for these types. Instead, formulators should choose a given grade based on the API characteristics and disintegration required. | |
Sodium starch glycolate can be added extragranularly, intragranularly, or as a 50:50 split between extragranular and intragranular additions. The physical properties of Sodium starch glycolate, and hence its effectiveness as a disintegrant, are affected by the degree of cross-linkage, the extent of carboxymethylation, and purity. There is evidence of brand-to-brand variability, probably due to differences in particle size, degree of substitution, and level of cross-linking.
When developing ODT formulations, it may be necessary to use superdisintegrants at even higher levels (10% or higher) in order to achieve rapid disintegration. At these levels, the superdisintegrant can have a significant impact on the formulation’s flowability, compactability, core strength, and the tablet’s mouth-feel.
Depending on the level and characteristics of the active pharmaceutical ingredient (API) and the desired release profile, the levels of superdisintegrant used can be 10–20 wt % of the formulation, and it can be higher or lower in some cases. Thus, in developing an ODT formulation for direct compression, choosing the optimal superdisintegrant is critical. Therefore, these factors must be carefully considered when selecting a superdisintegrant to use.
Interesting Fact
The term “super” as used in superdisintegrants was coined in the 1960s. It refers to the powerful disintegration force generated at low concentrations by this class of disintegrants, compared to traditional disintegrants.
Finally, there are several speciality grades of sodium starch glycolate available, including low pH (EXPLOTAB® Low pH, GLYCOLYS® Low pH), low viscosity (EXPLOTAB® CLV, GLYCOLYS® LV), low solvent (VIVASTAR® PSF), and low moisture GLYCOLYS® LM.
When used as a pharmaceutical excipient, Sodium starch glycolate is considered a safe and non-hazardous substance. Although not GRAS listed, it is regarded as non-toxic and non-irritating. However, it is a dust hazard, and irritation of the eyes or nasal passages may occur unless adequate precautions are in place to prevent dust generation.
The US Environmental Protection Agency has determined that Sodium starch glycolate does not have any significant toxic potential through ingestion. It is not absorbed in the intestinal tract in significant amounts.
Due to moderate inhalation toxicity concerns, health agencies recommend wearing dust masks when using this raw material in pharmaceutical industrial settings. There is no direct harm caused by inhalation to the general public when using this ingredient in pharmaceutical products. Sodium starch glycolate, alone or in combination with other substances, does not result in cumulative adverse effects.
Overall, given the low toxicity of Sodium starch glycolate, daily allowable intake has not been set because it poses no appreciable risks to human health under reasonably the current use scenarios.
Sodium starch glycolate is a stable formulation ingredient, although very hygroscopic. It should be stored in a dry place away from direct moisture in a well-closed container in order to protect it from wide variations in humidity and temperature, which may cause caking. If stored correctly, the physical properties of Sodium starch glycolate will remain unchanged for up to 3 years. Sodium starch glycolate is incompatible with ascorbic acid.
Sodium Starch Glycolate is sourced from potatoes and it is 100% biodegradable. It does not accumulate in the environment and has no long-term impact on ecology or marine life. Sodium Starch Glycolate excipient grade achieved a total score of 74/100 by the Excipients Forum Sustainable Chemistry Score.™
[1] E.M. Rudnic, J.L. Kanig, C.T. Rhodes, Effect of Molecular Structure Variation on the Disintegrant Action of Sodium Starch Glycolate, Journal of Pharmaceutical Sciences, 74 (1985) 647-650. https://doi.org/10.1002/jps.2600740613. Pubmed | Google Scholar
[2] S. Edge, A.M. Belu, U.J. Potter, D.F. Steele, P.M. Young, R. Price, J.N. Staniforth, Chemical characterisation of Sodium Starch Glycolate particles, International Journal of Pharmaceutics, 240 (2002) 67-78. https://doi.org/10.1016/S0378-5173(02)00109-6. Pubmed | Google Scholar
[3] U. Shah, L. Augsburger, Multiple sources of Sodium Starch Glycolate, NF: Evaluation of functional equivalence and development of standard performance tests, Pharmaceutical Development and Technology, 7 (2002) 345-359. https://doi.org/10.1081/PDT-120005731. Pubmed | Google Scholar
[4] P.M. Young, S. Edge, J.N. Staniforth, D.F. Steele, R. Price, Interaction of moisture with Sodium Starch Glycolate, Pharmaceutical Development and Technology, 12 (2007) 211-216. https://doi.org/10.1080/10837450601168763. Pubmed | Google Scholar
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.