Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
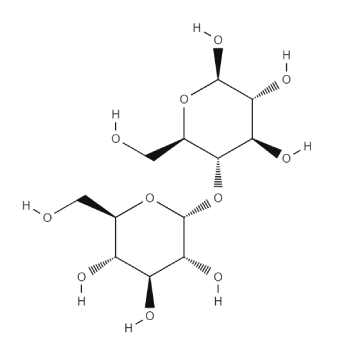
Maltodextrin is a saccharide-based pharmaceutical excipient consisting of a mixture of polymers of D-glucose residues, with a dextrose equivalent (DE) less than 20. The D-glucose units are linked primarily by α-(l → 4) bonds but there are branched segments linked by α-(1 → 6) bonds too. It is prepared by the partial hydrolysis of Starch with suitable acids and/or enzymes and is supplied as a white powder.
Pharmacopoeial Compliance: USP-NF; Ph.Eur; J.P; B.P
Synonyms and Trade Names: Maltodextrin; C* PharmDry®; Glucidex®; Glucodry®; Lycatab® DSH; Maldex®; Maltosweet®; Maltrin®; Paselli® MD10® PH; Star-Dri®
Uses and Applications: Tablet and Capsule Diluent, Tablet Binder, Coating Agent, and Viscosity- Increasing Agent
Maltodextrin is a special class of Starch excipient derivatives consisting of mixtures of oligosaccharides (short chains polymers of glucose linked primarily by α-1,4 glucosidic bonds). It is manufactured by enzyme catalysed hydrolysis of a starch source with or without acid. Maltodextrin is available in different molecular weights owing to the diversity of glucose polymers in the material. For this reason, Maltodextrin is often specified by the degree of polymerisation (DP), as well as the dextrose equivalent (DE), which is a measure of the percentage of reducing sugars in the maltodextrin relative to the percentage in glucose (which is 100%). Thus, Maltodextrin grades with a high DE have a lower DP and vice versa. DP and DE depend on the method of manufacture.
Note that there are different grades of Maltodextrin depending on the DE: those ranging from a DE value of 10-14, and those ranging from 15-19. Grades with a high DE values have greater sweetness and solubility but lower heat resistance while grades with lower DE values are only moderately sweet and best suited as bulking agents. The USP-NF does not set a specific DE value, and defines Maltodextrin simply as a “saccharide consisting of mixture of polymers that consist of D-glucose units, with a dextrose equivalent (DE) less than 20. The D-glucose units are linked primarily by α-(l → 4) bonds but there are branched segments linked by α-(1 → 6) bonds. It is prepared by the partial hydrolysis of a starch with suitable acids and/or enzymes”.
The process of producing Maltodextrin involves heating and treating starch with acid and/or enzymes in the presence of water. This process partially hydrolyzes the starch, to produce a solution of glucose polymers of varying chain length. This solution is then filtered, concentrated, and dried to obtain maltodextrin.
Various different grades of maltodextrin are commercially available for food and pharmaceutical applications from a number of suppliers: e.g. Lycatab® DS (Roquette Freres), Maltrin® (Grain Processing Corp.) and Star-Dri® (Tate & Lyle). The grades have different physical properties such as solubility and viscosity, depending upon their DE value.
Maltodextrin is supplied as a white, free-flowing powder having a moderate to bland taste.
| Chemical Name | Maltodextrin |
| CAS Registration Number | [9050-36-6] |
| Empirical Formula | (C6H10O5)n.H2O |
| Molecular weight | 900 – 9 000 |
| EINCES Number | 232-940-4 |
| UNII Code (FDA) | 7CVR7L4A2D |
Maltodextrin is an approved pharmaceutical excipient. It is currently listed in all the major pharmacopoeias and is also GRAS listed. It is included in the FDA Inactive Ingredients Database (tablets and granules). A specification for maltodextrin is contained in the Food Chemicals Codex (FCC).
| Form | Solid |
| Appearance | White powder or granules |
| Angle of repose | 26-36o |
| Bulk density | 0.10 – 0.65 g/cm3 |
| Tapped density | 0.30 to 0.45 g/cm3 |
| Moisture content | Hygroscopicity varies with DE. At <50% relative humidity, it is only slightly hygroscopic. |
| Particle size distribution | Available in various particle sizes depending on grade |
| Solubility | Freely soluble in water but slightly soluble in Ethanol (9.5%). |
| Specific surface area | 0.30-0.55 m2/g (typical) |
| Viscosity (dynamic) | <20 mPas (20 cp) for a 20% w/v aqueous solution of Lycatab® DSH. The viscosity of maltodextrin solutions decreases as the DE increases |
| USP-NF | Ph.Eur | |
| Name | Maltodextrin | Maltodextrin |
| Authorised use | Excipient | Excipient |
| Definition | specified | specified |
| Characters | White or almost white, free-flowing powder | White or almost white, free-flowing powder |
| Identification | A, B, C, D | A, B, C, D |
| pH | 4.0- 7.0 | 4.0- 7.0 |
| Sulphur dioxide | ≤40ppm | ≤20ppm |
| Dextrose Equivalent (DE) | <20.0 | 17.0 – 21.0 |
| Heavy Metals | ≤5 ppm | ≤10ppm |
| Protein | ≤0.10% | n/a |
| Residue on ignition | ≤0.50% | ≤0.5% |
| Loss on Drying | ≤6.0% | ≤6.0% |
| Sulphated Ash | n/a | ≤0.5% |
| Microbial contamination
TAMC & TYMC |
specified | specified |
| Assay | n/a | n/a |
| Labelling | specified | specified |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
In pharmaceutical products, Maltodextrin functions as a tablet and capsule diluent, tablet binder (wet and dry granulation), coating agent, and viscosity-increasing agent.
As an excipient in tablet formulations, Maltodextrin functions as a binder and diluent in both direct-compression and wet-granulation processes. It has been demonstrated that Maltodextrin has a minimal impact on dissolution rates even when lubricants such as Magnesium stearate (0.5-1.0%) are used in formulations.
Maltrodextrin grades with a high DE value are reportedly beneficial in the development of chewable tablets. It has also been added to oral liquid formulations to increase the viscosity of solutions and to prevent the crystallization of syrups during production or storage (cap lock phenomenon).
Maltrodextrin has also been used as a tablet film former in aqueous film-coating processes. it functions either as a primary or secondary film former.
Maltodextrin is reported to be a suitable carrier in spray-dried redispersible oil-in-water emulsions intended to improve the bioavailability of poorly soluble drugs.
Maltodextrin is also widely used in confectionery and nutraceutical/food products, as well as cosmetics and personal care products.
Therapeutically, maltodextrin is often used as a carbohydrate source in oral nutritional supplements because solutions with a lower osmolality compared with dextrose solutions. Maltodextrin solutions provide a higher caloric density compared with other sugars.
Since Maltodextrin is part of the carbohydrates food group of substances, it is considered a safe, non-irritant and nontoxic material. When ingested, it is readily digested and has a nutritional value of approximately 17kJ/g (4 kcal/g). There have been no safety concerns reported and it can be used in infant formula and to replace fat in many food products. However (in the European Union), Maltodextrins must be declared on the label.
Maltodextrin is a stable excipient. The standard shelf life is given at at least 12 months when the material is correctly stored (at a cool temperature, <300C and less than 50% relative humidity). However, Maltodextrin solutions may require the addition of an antimicrobial preservative. When storing the material long-term, Maltodextrin should be stored in a well-closed container in a cool, dry place.
Under certain pH and temperature conditions, maltodextrin may discolour (undergo Maillard reactions) on contact with amino acids groups.
When handling Maltodextrin in a work setting, you should observe prevailing SHEQ protocols appropriate to the circumstances and quantity of material handled. Eye protection is recommended. Maltodextrin should he handled in a well-ventilated environment and excessive dust generation should be prevented.
Maltodextrin is an excipient of plant origin derived from starch (maize, potato or wheat). In the EU, manufacturers use non-GMO varieties. It is also an inert and non-toxic excipient and considered safe for the environment, with minimal long-term impact on ecology or marine life. Maltodextrin excipient grade achieved a total score of 84/100 by the Excipients Forum Sustainable Chemistry Score™.
Tereos Starches and Sweeteners
Omnia Nisasta
Grain Processing Corporation
Tate & Lyle
[2] S. Nath, Y.V. Pathak, Evaluation of maltodextrins as tablet excipients I. Micromeritic and compressional characterization, Powder Technology, 75 (1993) 97-101.
[5] D.E. Wurster, S. Likitlersuang, Y. Chen, The influence of magnesium stearate on the Hiestand Tableting Indices and other related mechanical properties of maltodextrins, Pharmaceutical Development and Technology, 10 (2005) 461-466.
[6] G.K. Bolhuis, N. Anthony Armstrong, Excipients for direct compaction—an update, Pharmaceutical Development and Technology, 11 (2006) 111-124.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.