A pharmaceutical film coating is a polymer-based membrane applied onto solid dosage forms, such as tablets, capsules and granules, in order to impart certain desired attributes that are otherwise absent in the uncoated product.
In this post, we’ll tell you what film coatings are, why they are used in pharmaceutical products and how they are applied onto solid dosage forms.
What is a pharmaceutical film coating?
The term ‘coating’ usually implies a membrane formed directly on a substrate. It separates two phases between which fluids (gases, vapours or liquids) may be exchanged.
It is distinguished from ‘film’, a term used interchangeably with coating, which, however, refers to a preformed, stand-alone membrane (a film becomes a coating when applied onto a substrate) (Mwesigwa, 2006).
When used in relation to solid dosage forms, a film coating can be defined a polymer-based thin membrane applied onto the surface of tablets, capsules, granules and spheroidal unit dosage forms.
The coating generally imparts certain desirable attributes to the dosage that are otherwise absent in the uncoated form.
Such attributes include impartation of colour and branding marks, alteration of drug release properties, protection of sensitive ingredients or masking offensive odours or tastes, among others.
Pharmaceutical coatings are usually dense, non-porous films obtained from polymeric substances and are typically ≤ 100 μm thick (Hogan, 1995) although films with much higher thickness are not atypical.
Note that the use of polymer film coatings is not limited to solid dosage forms: many other products are routinely encased in polymer films of various types. For example, medical devices are coated with silicone resins to provide lubricity and protection.
Types of polymer film coatings
The practice of coating solid dosage forms (tablets, capsules, granules and spheroids) is a very old practice that has been undertaken for several centuries.
There are references to the use of mucilages to coat pills in Greco-Arab and Islamic herbal medical scripts.
As sugar became more widely available in the 19th century, the food and confectionery industry developed pan coating as a means for improving aesthetics of candy.
This technology was subsequently borrowed and adapted for use in the pharmaceutical industry, and remained the only means for coating until the early 1950 when the American pharmaceutical company, Abbott Laboratories marketed the first polymer film coated tablet.
This set a precedent for further introduction of many other polymers for use in pharmaceutical coatings. Presently, although sugar coated drug products are still widely marketed the vast majority of products utilise polymer film coatings.
Polymer coatings can be broadly categorized into three groups based on their functional roles:
(1) Conventional coatings
Conventional coatings are sometimes known as non-functional or immediate release coatings.
They are the most commonly encountered type of tablet coating.
The reason they are known as non-functional coatings is because they are designed to dissolve rapidly in the gastrointestinal tract without any significant impact on the rate of drug release in the gut.
Conventional coatings serve several purposes, including:
- protective
- aesthetics
- masking taste or odours
- improving mechanical strength of the core
- facilitating product handling
- reduction of the risk of interaction between incompatible components, and
- reduction of dust and the associated explosion hazards
The main requirement for conventional coatings is pH independent solubility, as mentioned above, so as to have a minimum influence on drug release of the core.
(2) Delayed release coatings
Delayed release coatings are mainly of the enteric type.
They are designed to prevent disintegration of the dosage form in the stomach. However, on reaching the intestine, delayed release coatings disintegrate to release the drug for absorption or local action.
There are many reasons why an enteric coating may be applied to solid dosage forms:
- Firstly, they may be utilised to protect certain drug substances which are hydrolysed in the stomach by gastric secretions (e.g., acids and enzymes),
- They can be used to prevent gastric distress associated with acidic drugs (e.g., aspirin or other NSAIDs),
- They can help deliver drugs intended for local action in the intestines, e.g., intestinal antiseptics, to deliver drugs that are optimally absorbed in the intestines to their prime absorption site, or to effect a delayed response to the dosage form.
Therefore, the ideal enteric-coating should be impermeable to gastric juices but easily susceptible to intestinal juices.
The majority of enteric polymers are have carboxyl ionizable groups that become protonated in acid media but form salts in alkaline pH enabling them to dissolve in the intestinal juices.
(3) Sustained release coatings
The third category is for covers coatings used to control the rate of release of drug substances from a dosage form and extend the release time over several hours.
Sustained release coatings are also known as controlled, extended or prolonged release coatings, although teach of these terms has specific meanings.
Generally, the most common mechanism of sustaining release is controlling the diffusion rate of the drug through the polymer membrane.
Depending on the diffucion mechanism sought, different release kinetics can be obtianed, such as zero-order (constant release), first-order release kinetics, or pulsatile or sigmoid release patterns.
Note that in the literature, the term ‘modified release’ coating is also sometimes used. This term is non-specific and covers both delayed release and sustained release coatings.
Controlled release coatings serve a variety of purposes:
- They are used to achieve more effective therapy while eliminating the potential for both under- and overdosing.
- Controlled release coatings are also used to achieve the slow release of highly soluble and or permeable drugs, fast release of low-solubility drugs, or delivery of drugs to specific sites, such as the colon.
- Lastly, it is not uncommon that pharmaceutical companies to use controlled release technologies as a way of achieving brand line extension or to add marketing depth to an existing brand.
Materials used in polymer film coatings
A film coating is constituted from a variety of materials that include a polymer, a plasticizer, and other additives (surfactant, colorants/pigments and lubricants).
Once combined and dissolved or dispersed in a solvent or aqueous medium, the different ingredients are sprayed onto the substrate and allowed to form a thin, continuous envelope.
Polymers
The polymer is the film former and the main component on the formulation.
The ideal polymer is one capable of producing smooth, thin films that are reproducible under routine coating conditions.
The range of materials that can be used as film formers is very wide and covers materials that may be natural, synthetic or semi-synthetic.
Polymers used in conventional film coatings
This category includes:
- cellulose ether derivatives, e.g., hypromellose (also called hydroxypropyl methylcellulose), methylcellulose, hydroxypropylcellulose and sodium carboxy methylcellulose
- vinyl polymers, e.g., polyvinyl alcohol and polyvinylpyrrolidone
- polymethacrylates, mainly copolymers of butylmethacrylate, (2-dimethylaminoethyl) methacrylate and methylmethacrylate
- Other polymers e.g., zein, modified starches and polysaccharides
Polymers used in delayed release film coatings
- This category mainly includes enteric polymers such as:
- polymethacrylate derivatives (e.g., ethylmethacrylate copolymer)
- polyvinyl acetate derivatives, e.g., poly(viny acetate phthalate), and
- cellulose derivatives, e.g., cellulose acetate phthalate, cellulose acetate trimellitate, hydroxypropyl methylcellulose acetate phthalate, and hydroxypropyl methylcellulose acetate succinate.
Polymers used in extended/sustained-release film coatings
This category includes the following compounds:
- cellulosic derivatives, e.g., ethylcellulose and cellulose acetate
- polyacrylates, such as Eudragit R series of polymers; and
- polylactides, poly(lactide-co-glycolides and polyorthoesters
Plasticizers
Plasticizers are low molecular weight organic esters added to coatings to facilitate processing.
They modify generic properties of the polymer rendering them easy to process and or to perform as membranes.
Although many compounds, covering diverse functions like phthalates, aliphatics, epoxides (vegetable oils), etc., and even polymers, possess plasticizing properties, only few are approved for pharmaceutical use.
These include polyols such as glycerol, propylene glycol and polyethylene glycols, organic esters such as phthalates, dibutyl sebacate, and triacetin, vegetable oils/glycerides such as castor oil.
Miscellaneous additives
Additives serve a variety of purposes: Colorants are used for individualization of the product by colour.
They may be pigments, e.g., amaranth, γ-carotene, or dyes such as lakes of aluminium or opacifiers, which are used to impart opacity. Talc and titanium dioxide are mainly used opacifiers.
Fillers and anti-tack agents are used to provide coating solids to the formulation and to reduce tackiness.
They include talc, microcrystalline cellulose, glyceryl monostearate, stearic acid and magnesium stearate.
Solvents
Solvents permit uniform film formation and application over the entire substrate.
Those which have been used for film coating include water, ethanol, ethanol/water, and various other solvent/solvent mixtures.
Their selection is based on the following criteria:
(i) empirically; according to the “like dissolves like” rule;
(ii) qualitatively, based on the assessment of intermolecular forces; and
(iii) quantitatively, based on solubility parameters
Up to the early 1970s, organic solvents were the main stay of film-coating applications. However, due to increasing costs, concerns about toxicity and effects on the environment, a move to aqueous based systems was prompted.
Therefore, water is the preferred coating medium today although a few companies still use organic solvents.
How Film Coatings Are Applied
Film coating of solid dosage forms in the pharmaceutical industry is undertaken in a specialised coating equipment, such as solid coating pans, perforated pans or fluidised coater.
The process involves loading the cores into the pan, which is spun at a low to moderate speed, while a stream of hot air is pumped into the bed of rotating cores.
A solution or dispersion of the coating (polymer, plasticizer, pigment, etc) is then pumped and sprayed into the moving bed at a pace matched with the rate of evaporation of the solvent, leading to the formation of a thin film on the core surface.
This is illustrated in the infographic below:
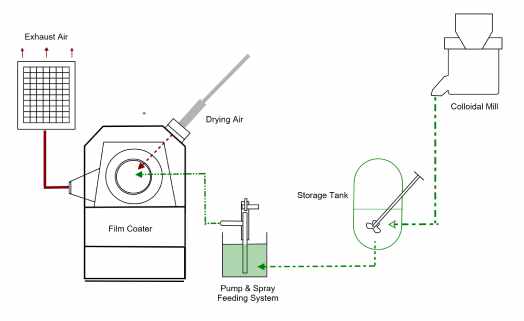
The different steps involved in a typical tablet film coating, as it is carried out presently, can be split into several stages:
- Preparation of the coating dispersion
This step is required in order to effectively transform coating components into a medium that can be efficiently applied onto the coating substrates.
Typically, the coating materials are dissolved or dispersed in a water, water-alcohol solutions, acetone or ethyl acetate. Organic solvents are rarely used due to the potential hazards from flammability and toxicity.
- Droplet generation/atomization and transfer onto substrates
Atomization is the process of breaking up the coating liquid into a spray of droplets. In film coating, atomization allows the polymer coating to be efficiently and uniformly transferred onto the substrate.
The main mechanism of atomization involves pumping the coating fluid through a pneumatic nozzle. As the liquid emerges at the nozzle tip the air stream that surrounds it disrupts its flow to create a fine mist.
- Droplet drying, coalescence and adhesion
Upon contact with the substrates, heat and mass transfer take place between the droplets, heated air and the substrate. Water evaporates from the droplets and is transferred into the air by convection. The solid payload deposits onto the surface, adheres and dries on the surface to create a film.
The choice between water and solvent-based coatings
Even though organic solvents are much easier to process, there has been a steady move away from their use in pharmaceutical coatings due to various reasons.
- The different reasons include:
- rising costs of solvents
- hazards and risks due to flammability
- regulations
- environmental concerns
These factors, together with the availability of better designed equipment and introduction of high performance, fully-formulated coating systems, mean that aqueous coatings are by far the most widely used systems today.
Examples of Film Coating Formulae
The following are gexamples of film coating formulae based on the concepts outlined above:
Convetional coatings
High gloss coating formula
| Material | % w/w | Function |
|---|---|---|
| Sodium carboxymethylcellulose | 40 | Film former |
| Maltodextrin | 18 | Extender |
| Dextrose | 15 | Extender |
| Lecithin | 10 | Surfactant/dispersant |
| Sodium citrate | 2 | Stabiliser |
| Pigments & oxides | 10 | Pigments |
| Colours & dyes | 5 | Colorants |
General purpose coating (PVA-based)
| Material | % w/w | Function |
|---|---|---|
| Polyvinyl alcohol | 40 | Film former |
| Polyethylene glycol 3350 | 20.2 | Plasicizer |
| Talc | 14.8 | Antiadherent |
| Pigments | 25 | Colorants |
General purpose (HPMC-based)
| Material | % w/w | Function |
|---|---|---|
| HPMC 606 | 40 | Film former |
| Lactose monohydrate | 12 | Extender |
| Polyethylene glycol 3350 | 18 | Plasticizer |
| Triacetin | 5 | Plasticizer |
| Pigments | 25 | Colorants |
Enteric coating
| Material | % w/w | Function |
|---|---|---|
| Methacrylic acid Copolymer Dispersion (30% aqueous dispersion) | 16.6 | Enteric polymer film former |
| Talc | 4 | Antiadherent |
| Polyethylene Glycol 6000 (10% squeous solution | 1.6 | Plasticizer |
| Antifoam emulsion | 0.2 | Foam suppressant |
| Water | 77.6 | Solvent |
Summary about Pharmaceutical Film Coatings
The oral route of drug administration is the most popular means by which people take medication.
It allows the development of immediate and modified-release products with unparalleled ease.
One of the commonest means of modifying drug release in oral dosage forms is the use of polymeric film coatings. Polymers are selected for this purpose because they are able to form thin membranes or coatings that are not only strong but can also impart many useful properties to the dosage form.
It is also on the basis of the functional basis of polymer coatings that they are classified, thus, we have conventional or non-functional coatings, extended release (enteric coatings) and controlled release coatings.
Sources
Pharmacentral has a strict referencing policy and only uses peer-reviewed studies and reputable academic sources. We avoid use of personal anecdotes and opinions to ensure the content we present is accurate and reliable.
- Rhodes, C. T., Porter, S. C (1998). Coatings for controlled-release drug delivery systems. Drug Dev. Ind. Pharm., 34, 1139-1154.
- Nagai, T., Obara, S., Kokubo, H., Hoshi, N (1997). Application of HPMC and HPMCAS to aqueous film coatings of pharmaceutical dosage forms. In: McGinnity, J. W., (Ed.), Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms, vol. 7, 2nd Ed., Dekker, New York.
- Hogan, J. E (1995). Film coating materials and their properties. In: Cole, G., Hogan, J. E., Aulton, M. , (Eds.), Pharmaceutical Coating Technology, Taylor and Francis Ltd, London, pp. 6-50.
- Mwesigwa, E (2006). Sorption and Permeation of Moisture in Moisture Barrier Polymer Film Coatings, PhD Thesis, University of London.