Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
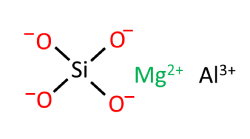
Magnesium Aluminium Silicate is either a naturally occurring mineral clay or an artificially synthesised complex mineral excipient composed of magnesium, aluminium, silicon, oxygen, and water. It is chemically described as a polymeric complex made up of layers of alumina and silica sheets. Other elements may be present, such as iron, lithium, calcium and carbon. It is supplied in the form of an off-white to creamy-coloured, odourless and tasteless micronised or flaky powder.
Pharmacopoeial Compliance: USP-NF; Ph.Eur; B.P
Synonyms and Trade Names: Aluminium Magnesium Silicate; Magnesium Aluminum Silicate; Colloidal Magnesium aluminosilicate; Silicic Acid Aluminum Magnesium salt; Carrisob®; Gelsorb®; Magnbrite®; Neusilin®; Pharmasorb®; Veegum®
Uses and Applications: Adsorbent; Stabilising Agent; Tablet and Capsule Disintegrant; Tablet Binder, and Viscosity-Increasing Agent
Magnesium aluminium silicate is a complex mineral compound composed of magnesium, aluminium, silicon, oxygen, and water in varying proportions. It is used widely as a pharmaceutical excipient as well as an adsorbent in cosmetic formulations and may be obtained by refining naturally-occurring smectite clays, mainly montmorillonite and saponites or created synthetically from Al, Si and Mg.
In the USP-NF, Magnesium aluminium silicate is defined as a mixture of colloidal montmorillonite and saponite that has been processed to remove impurities. In this compendia, four types of magnesium aluminium silicate are listed: IA, IB, IC, and IIA, which differ according to viscosity and ratio of aluminium and magnesium content. In contrast, the Ph.Eur describes Magnesium aluminium silicate as a mixture of particles with colloidal particle size of montmorillonite and saponite, free from grit and non-swellable ore.
In the natural form, Magnesium aluminium silicate generally has an atomic lattice consisting of two structural units: an octahedral unit consisting of two sheets of closely packed oxygens or hydroxyls, with Al, Fe or Mg atoms embedded in these sheets in an octahedral configuration so that they are equidistant from the oxygen or hydroxyl groups. The mineral composition of naturally occurring Magnesium aluminium silicate has been determined as follows: Silicon dioxide 61.1%, Magnesium oxide 13.7%, Aluminium oxide 9.3%, Titanium dioxide 1.1%, Ferric oxide 0.9%, Calcium oxide 2.7%, Sodium oxide 2.9%, Potassium oxide 0.3%, Carbon dioxide 1.8%, and water of combination 7.2%. It also has trace amounts of F, MnO, and ZnO.
The totally synthetic form of Magnesium aluminium silicate is an amorphous form and corresponds to the chemical formula, Al2O3. MgO. 1.7SiO2. xH2O. It contains either the tetrahedron or octahedron of Al, octahedrom of Mg or tetrahedron of Si, which are randomly linked into a complex 3-D structure. Synthetic Magnesium aluminium silicate has the following composition: Al2O3. (29.1-35.5%); MgO (11.4-14.0%) and SiO2 (29.2-35.6%). Synthetic Magnesium aluminium silicate is marketed under the trade name, Neusilin® (Fuji Chemical).
Excipient-grade Magnesium aluminium silicate is supplied as off-white to creamy white, odourless, tasteless soft, slippery small flakes, or as a fine, micronized powder.
| Chemical Name | Aluminium Magnesium Silicate |
| CAS Registration Number | [12511-31-8] |
| Empirical Formula | MgAl2(SiO4)2 |
| Molecular weight | 143.369 |
| EINCES Number | 235-682-0 |
| UNII Code (FDA) | 7TO1453RTL |
| Examples | Neuslin |
| Chemical Name | Magnesium Aluminium Silicate |
| CAS Registration Number | [1327-43-1] |
| Empirical Formula | Mg2Al2F30Si5 |
| Molecular weight | 482.99 |
| EINCES Number | 215-478-8 |
| UNII Code (FDA) | 6M3P64V0NC |
| Examples | Magnabrite; Vangel; Attagel 20; Veegum |
Magnesium aluminium silicate is listed in the PhEur and USP-NF. It is also included in the FDA Inactive Ingredients Database (oral granules, solutions, suspensions and tablets; rectal; and topical preparations; vaginal preparations).
| Form | Solid |
| Appearance | White to off-white powder |
| Solubility | Insoluble in water and organic solvents |
| pH | 9.0-10.0 (5% aqueous dispersion) |
| Melting point | 900 oC |
| Density | 2.418g/cm3 |
| Moisture content | 6.0 – 98% |
| Swelling capacity | Swelling properties are reversible. Magnesium aluminium silicate swells to many Limes its original volume in water to form colloidal dispersions, and may be dried and rehydrated any number of times |
| Viscosity | Rheological behaviour is reported as follows: At concentrations of 1-2% w/v, Magnesium aluminium silicate forms thin colloidal suspensions. At 3 % w/v, opaque dispersions are formed. At ≥3 % w/v viscosity of aqueous dispersions increases rapidly, to form thick, white colloidal sols (thixotropic). At 10% w/v gels are formed. Dispersions are thixotropic at concentrations greater than 3% w/v |
| USP-NF | PhEur | |
| Official name | Magnesium aluminium silicate | Aluminium magnesium silicate |
| Authorised use | Excipient & API | Excipient and API |
| Definition | specified | specified |
| Identification | specified | specified |
| Characters | n/a | specified |
| Viscosity (5% w/v suspension) | By Type | n/a |
| Microbial limits | 103 cfu/g | 103 cfu/g |
| pH (5% w/v suspension) | 9.0 – 10.0 | 9.0 – 10.0 |
| Acid demand | n/a | specified |
| Loss on drying | 8.0% | 8.0% |
| Arsenic | 3 ppm | 3 ppm |
| Lead | 0.0015% | 15 ppm |
| Assay for Al and Mg content | specified | 95.0 – 105.0 |
| Labelling | specified | n/a |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Magnesium aluminium silicate is a versatile pharmaceutical excipient that is currently used as an adsorbent, stabilizing agent, suspending agent, tablet and capsule disintegrant, and thickener (viscosity-increasing agent) in a variety of dosage forms. It has been used over several decades to formulate tablets, ointments, and creams taking advantage of its unique physicochemical properties, i.e swelling, thickening, surface area and/or porosity). The functionality to which it is applied depends on whether the Magnesium aluminium silicate is naturally-derived or purely synthetic.
In liquid oral products, naturally-occurring Magnesium aluminium silicate is used as a suspending and stabilizing agent either alone or in combination with other suspending agents. When used in combination with other viscosity suspending agents, such as Xanthan gum, the performance of Magnesium aluminium silicate is greatly improved owing to synergistic effects. Synthetic Magnesium aluminium silicate possesses no thickening or gelling properties and is not used for this purpose.
In oral tablets, both natural and synthetic Magnesium aluminium silicate grades can be used as binders and disintegrants in conventional tablets or as active pharmaceutical ingredient release modifiers. Synthetic Magnesium aluminium silicate exhibits a large specific surface area, high porosity and high water and oil-absorbing capacity, making it an ideal absorbent material for converting oils into compressible powders without a loss in compactibility.
Typical application levels are summarised in the table below:
| Application | Typical Level (%) |
| Oil or water adsorbent | 10-50 |
| Binder (tablets and granules) | 2-10 |
| Stabilising agent for oral emulsions | 2-10 |
| Stabilising agent for topical emulsions | 1-5 |
| Viscosity-increasing/suspending agent (topical) | 1-10 |
| Dental care (toothpaste) as a gelling agent | 0.5-3 |
| Viscosity modifying agent | 2-10 |
Speciality-grades of synthetic Magnesium aluminium silicate have excellent antacid activity and can be used to protect the stomach from hyperacidity and prevent ulcer formation. It can rapidly neutralize excessive gastric acid and control pepsin activity by adsorption. Moreover, it can also adhere to and protect the stomach wall from the effects of hazardous substances.
Magnesium aluminium silicate is an approved excipient that is generally regarded as a safe, nontoxic and nonirritating substance when used at the concentrations typically employed in pharmaceutical products. Studies in rats and dogs fed Magnesium aluminium silicate (at 10% of the diet for 3 months), including postmortem and histopathological examinations, were negative. The safety of Magnesium aluminium silicate has also been evaluated by the Cosmetic Ingredient Review Expert Panel, which found that while solid particles could be irritating eyes, topical applications did not result in any remarkable changes to human skin. It is neither irritating nor sensitizing, and is absorbed by the skin.
Toxicology: LD50 (rat, oral): > 16g/kg
A pharmacological and toxicological report on Magnesium aluminium silicate by Fuji Chemical can be found through this link: Pharmacological, toxicological and clinical data of Neusilin and Neusilin A: Fuji Chemical Industries Report
Magnesium aluminium silicate is stable when stored under dry conditions. The shelf life is stated as 36 months. It is stable over a wide pH range, although it exhibits base-exchange capacity, with the capacity to absorb a number of organic substances. For this reason, Magnesium aluminium silicate should be stored in a well-closed container, in a cool, dry place. Magnesium aluminium silicate is reported, as with other clays, to likely sequester some active drug ingredients, which can result in low bioavailability.
When handling Magnesium aluminium silicate, workers should observe established institutional protocols for safe handling of chemicals appropriate to the quantities or the circumstances of the material being processed. The use of suitable eye protection and gloves is recommended. Furthermore, adequate ventilation should be in place and any risk of dust generation prevented.
A sustainability score for Magnesium aluminium silicate has not been computed. However, the excipient is naturally-obtained (from mineral ores) that are in plentiful supply.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.