Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Hypromellose is a mixed cellulose ether and a pharmaceutical excipient. It is obtained directly from naturally occurring plant cellulose fibres, which are partially etherified with methyl groups and a small proportion of hydroxypropyl groups. It is supplied as an odourless and tasteless, white or creamy-white fibrous or granular powder and is available in multiple grades that vary according to molecular weight, degree of substitution, and viscosity.
Synonyms & Trade Names: Hypromellose; HPMC; Hydroxypropyl Methylcellulose; E464; METHOCEL®; Methylcellulose Propylene Glycol ether; Methyl Hydroxypropylcellulose; METOLOSE™; PHARMACOAT™; TYLOSE® MO; AQUAT™; BENECEL™
Pharmacopoeia Conformity: USP-NF; Ph. Eur; JP; IP; FCC
Uses & Applications: Wet Granulation Binder; Suspending Agent; Viscosity-increasing agent; Solubiliser; and Matrix Forming Polymer
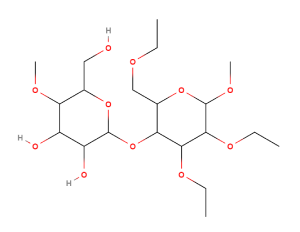
Hypromellose (also known as Hydroxypropylmethyl cellulose or HPMC) is a partially methylated and hydroxypropylated cellulose ether and an important pharmaceutical excipient. It is obtained directly from naturally-occurring plant fibres, and occurs as an odourless and tasteless, white or creamy-white fibrous or granular powder.
The basic monomer unit in the cellulose backbone as found in hypromellose is the β-D-glucose unit with a (1->4) linkage. The three free hydroxy groups are partially etherified with methyl (i.e -OCH3 or methoxy) groups. In addition, hydroxypropyl and methyl groups can be added to both the naturally-occurring hydroxyl groups of the cellulose and the newly formed (substituted) hydroxypropyl, i.e (-OCH2 CH (OH) CH3, hydroxypropyl) groups.
In the Ph.Eur, Hypromellose is described as a partly O-methylated and O-(2-hydroxypropylated) cellulose. Different grades are distinguished by viscosity and degree of substitution, usually by a number indicative of the apparent viscosity, in mPas, of a 2% w/w aqueous solution at 20 oC. On the other hand, the USP defines hypromellose in terms of the substitution type (by a four-digit number to the non-proprietary name: e.g
The first two digits refer to the approximate percentage content of the methoxy group while the last two digits refer to the approximate percentage content of the hydroxypropoxy group, calculated on a dried basis.
In some textbooks, hypromellose types are classified on the basis of the degrees of methoxy and/or hydroxyproyl substitution into three main groups, which correspond to the USP codes as shown below:
| Type | Letter Code | Methoxy Content % | Hydroxypropyl Content % |
| 1828 | C | 16.5-20.0 | 23.0-32.0 |
| 2208 | D | 19.0-24.0 | 4.0-12.0 |
| 2906 | D | 27.0-30.0 | 4.0-7.5 |
| 2910 | E | 28.0-30.0 | 7.0-12.0 |
It is not uncommon for some manufacturers to use their own codes for different grades of hypromellose. For example, Dupont (formely, Dow Chemical) uses codes E, F, and K, which principally indicate the degree of substitution.
In addition, the letter codes above may be accompanied by a number that indicates the viscosity of a 2% aqueous solution, usually multiplied by 100 as C or 1000 as M.
Some grades have additional acronyms that provide further information on the application of that particular grade. For instance, P stands for premium, LV for low viscosity, CR for controlled release, G for granular, or FG for food grade.
In the pharmaceutical sector, Hypromellose 2208, 2906 and 2910 are the grades most commonly used as binders, film coatings and matrix formulation. Hypromellose 1828 is also available, although not widely used in the development of dosage forms.
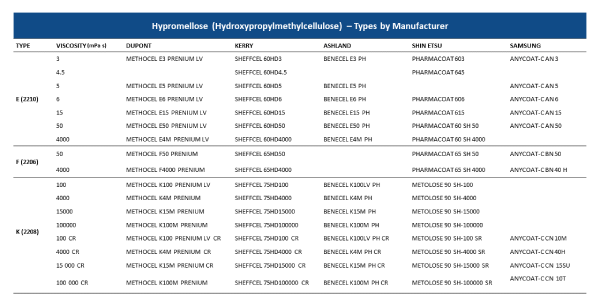
The table below provides a simple guide about the different grades of Hypromellose available from the different manufacturers:
Hypromellose is prepared from a purified form of cellulose, obtained from cotton or wood pulp, which is reacted with sodium hydroxide solution to produce swollen alkali cellulose. The alkali cellulose is then treated with chloromethane and propylene oxide to produce methyl hydroxypropyl ethers of cellulose. The fibrous reaction product is then purified and ground to a fine, uniform powder or granules. Hypromellose can then be exposed to anhydrous hydrogen chloride to induce depolymerization, yielding low viscosity grades.
Hypromellose has been used in pharmaceutical dosage forms for a long time. It is the most widely used polymer in non-functional premixed coating systems, such as Opadry, (Colorcon) and Advantia Prime Coating Systems (Ashland).
Some Hypromellose grades, such as METHOCELTM K4MP DC and METHOCELTM KI00MP DC (Dupont), have been developed and commercialized to enable direct compression modified-release tablets.
Powdered or granular, surface-treated grades of hypromellose are also available that are dispersible in cold water. These are not recommended for oral use.
To prepare an aqueous solution of Hypromellose, it is recommended that hypromellose be dispersed and thoroughly hydrated in about 20–30% of the required amount of water. Start with vigorously stirring and heating the water to 80–90 degrees Celsius, and then add the hypromellose. The heat source can be removed once the hypromellose has been thoroughly dispersed into the hot water. Sufficient cold water should then be added to produce the required volume while continuing to stir.
When aqueous/organic cosolvent mixtures are used for solution preparation, hypromellose should first be dispersed into the organic solvent at a ratio of 5–8 parts of solvent to 1 part of Hypromellose. Cold water is then added to produce the final volume.
| Chemical Name | Cellulose hydroxypropyl methyl ether |
| CAS Registration Number | [9004-65-3] |
| Empirical Formula | C29H54O20 |
| Molecular weight | 10 000 – 1 500 000 |
| EINCES Number | N/A |
| UNII Code (FDA) | 58779NIC8P |
Hypromellose is an approved pharmaceutical excipient. It is currently listed in the USP-NF, Ph.Eur and JP. Hypromellose is also GRAS listed and included in the US FDA Inactive Ingredients Database (covering ophthalmic and nasal products, oral tablets and capsules, oral liquids, and tablets; topical products). Hypromellose is also approved as a food additive in Europe (E464) and a specification for Hypromellose is contained in the Food Chemical Codex.
| Form | Solid, powder |
| Appearance | White or whitish fibrous or granular powder |
| pH (2% w/w aqueous solution) | 5.0-8.0 |
| pKa | n/a |
| Ash | ≤1.5% |
| Angle of repose | 35-44 |
| Gelling temperature | 40-90 oC |
| Auto ignition temperature | 360 oC |
| Relative density | 1.26 |
| Bulk density
Tapped density True density |
0.341g/ml
0.557g/ml 1.326 g/ml |
| Melting point. | Browns at 190-200 oC
Chars at 225 – 230 oC |
| Glass transition temperature | 170 – 180 oC |
| Moisture content | Absorbs moisture from the atmosphere; the amount absorbed depends upon the initial moisture content and the temperature and relative humidity of the surrounding air |
| Solubility | Soluble in cold water to form a viscous colloidal solution. Practically insoluble in hot water and ethanol (95%) but soluble in mixtures of water and alcohol |
| Viscosity (dynamic) | A wide range of viscosity types are commercially available. |
| Test | Specification | Reference |
| Identification | A, B, C, D, E, F | USP-NF/Ph.Eu/JP |
| Appearance | White or yellowish-white powder or granules | USP-NF/Ph.Eu/JP |
| Appearance of solution | + | USP-NF/Ph.Eu/JP |
| pH | 5.5 – 8.0 | USP-NF/Ph.Eu/JP |
| Chlorides | ≤0.5% | USP-NF/Ph.Eu/JP |
| Apparent viscosity | + | USP-NF/Ph.Eu/JP |
| Heavy Metals | ≤20pm | USP-NF/Ph.Eu/JP |
| Loss on drying | ≤5.0% | USP-NF/Ph.Eu/JP |
| Sulphated ash | ≤1.5% | USP-NF/Ph.Eu/JP |
| Assay | USP-NF/Ph.Eu/JP | |
| Methoxy content | 16.5-30.0 | USP-NF/Ph.Eu/JP |
| Hydroxypropoxy content | 4.0-32.0 | USP-NF/Ph.Eu/JP |
Hypromellose is a highly versatile excipient. It is widely used in oral, nasal, ophthalmic and topical pharmaceutical formulations as a:
Hypromellose is also used as an active agent in the formulation of artificial tears in the management of dry eye disease (keratoconjunctivitis sicca) and also Xerostomia.
In oral solid dosage forms, Hypromellose is primarily used as a tablet binder, in film-coating, and as a matrix for use in extended-release tablet formulations. In liquid dosage forms, it is used as a thickening and viscosity-increasing agent.
Concentrations between 2% and 5% w/w are used as binders in either wet or dry-granulation processes. For conventional wet granulation processes, Hypromellose 2910 with viscosities ranging from 3 mPa S to 15 mPa s is recommended.
High-viscosity grades may also be used, but they can inadvertently retard the release of drugs from tablets.
High viscosity and/or specialty controlled-release grades of Hypromellose 2910 and Hypromellose 2208 are used in the fabrication of matrices owing to their ability to gel and control drug diffusion and release. Typical usage levels are 10—80% w/w in tablets and capsules.
| Required polymer features | Grades | |
| Low solubility drugs | Moderate to Fast hydration to form gel layer for release control | Hypromellose 2906 and 2910 (e.g METOLOSE 65SH and 60 SH 1000 |
| Medium to high solubility drugs | Fast hydration to form gel layer for release control | Hypromellose 2208 (e.g METHOCEL® K100M) |
Hypromellose is also used in liquid oral dosage forms as a suspending and/or thickening agent at concentrations ranging from 0.25–5.0%. Any Hypromellose grade (2208, 2906, and 2910) is suitable for use in both low and high viscosities.
Compared with methylcellulose, hypromellose produces aqueous solutions of greater clarity with fewer undissolved fibers present, and is therefore preferred in formulation for ophthalmic use.
Hypromellose at concentrations between 0.45 – 1.0% w/w may be added as a thickening agent to vehicles for eye drops and artificial tear solutions. It is also used commercially in liquid nasal formulations at a concentration of 0.1%.
Hypromellose is used as a suspending agent and stabilizing agent in topical gels and ointments. As a protective colloid, it can prevent droplets and particles from coalescing or agglomerating, thus inhibiting the formation of sediments.
Hypromellose 2910 grades of low viscosity (5–15 mPa s) can be used as film formers in concentrations of 2–20% w/w.
Lower-viscosity grades are preferred for aqueous film-coating processes, while higher-viscosity grades are used with organic solvents.
Examples of hypromellose coating materials that are commercially available include AnyCoat A, Spectracel, Pharmacoat, and the Methocel E Premium LV series.
Hypromellose is also used as a suspending and thickening agent in topical formulations. In addition, hypromellose is used in the manufacture of capsules, as an adhesive in plastic bandages, and as a wetting agent for hard contact lenses.
Hypromellose is also widely used in cosmetics and food products.
Hypromellose has been used in pharmaceutical products for several decades and is generally regarded as a nontoxic and non-irritating material, although excessive oral consumption may have a laxative effect. The WHO has not specified an acceptable daily intake (ADI) for Hypromellose since the levels consumed were not considered to represent a hazard to health. In fact, high dosages of Hypromellose are being investigated for the treatment of various metabolic syndromes.
Toxicology: LD50 (mouse, IP): 5g/kg and LD50 (rat, IP): 5.2g/kg
Hypromellose powder is a stable material, although it is hygroscopic after drying. The assigned shelf life is 24-36 months. Hypromellose bulk powder should be stored in a well-closed container in a cool, dry place away from direct heat or moisture.
Solutions are reportedly stable at pH 3-11. Adequate preservation should be implemented if solutions are to be stored over a long time period in order to prevent mold formation and/or spoiling.
When heated and cooled, Hypromellose undergoes a reversible sol-gel change. The gelation temperature ranges from 50 to 90 degrees Celsius, depending on the grade and concentration of the material. At temperatures below the gelation temperature, the viscosity of the solution varies inversely with temperature (i.e decreases as the temperature is increased).
When handling Hypromellose, observance of SHEQ precautions appropriate to the circumstances and quantity of material handled is recommended. Hypromellose dust may be irritating to the eyes, thus, eye protection is also recommended. Excessive dust generation should be avoided to minimize explosion risks.
Hypromellose is a naturally-derived polymer obtained through the chemical modification of cellulose. It is an inert and non-toxic excipient and considered safe for the environment, with minimal long-term impact on ecology or marine life. Hypromellose excipient grade achieved a total score of 78/100 by the Excipients Forum Sustainable™ Chemistry Score.
[1]. M. Levina, A.R. Rajabi‐Siahboomi, The influence of excipients on drug release from hydroxypropyl methylcellulose matrices, Journal of Pharmaceutical Sciences, 93 (2004) 2746-2754. DOI: 10.1002/jps.20181. Pubmed.
[2]. A. Miranda, M. Millán, I. Caraballo, Study of the critical points of HPMC hydrophilic matrices for controlled drug delivery, International Journal of Pharmaceutics, 311 (2006) 75-81. DOI: 10.1016/j.ijpharm.2005.12.012. Pubmed
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.