As medical needs become more complex, the need for modified release pharmaceutical dosage forms becomes ever more salient. The matrix tablet technology, which uses matrix forming excipients, has been instrumental in this quest. But exactly what is a matrix former? How do they function, and how are they used? This technical note provides an overview, types and function of this important class of pharmaceutical ingredient.
What is a matrix forming excipient?
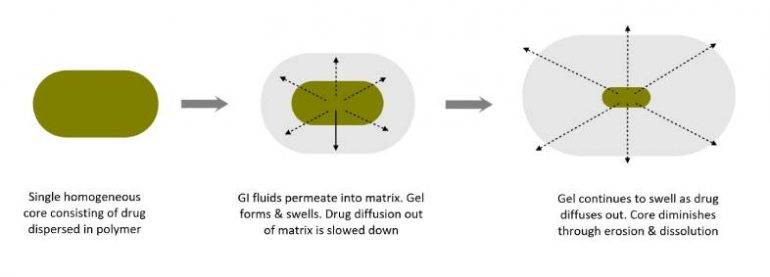
Matrix forming excipients are a class of materials used to fabricate sustained-release (prolonged release) pharmaceutical tablets. The matrix former creates a three-dimensional network that not only acts as a structural and functional scaffold for active ingredients but is the physical embodiment of the dosage form.
The matrix controls the entry of GI fluids into the tablet which retards the rate of diffusion of the active ingredient from the tablet, hence, its elimination half-life (time taken for the drug’s plasma concentration to reduce by 50%).
Matrix tablets have been around for more than half a century. Their benefits are easy to see when you consider the fact that 75 percent of all sustained release solid dosage forms used by patients today utilise matrix systems, a proportion that is larger than tablet film coatings, and many other approaches currently available for use to pharmaceutical scientists.
Why are matrix formers used in tablets?
The main reason for using tablet matrices is to achieve sustained release drug delivery. These dosage forms are designed to deliver active ingredients over a period of up to 24 hours. This results into longer-lasting therapeutic effects while reducing dosing frequency and side effects, while improving individual’s well-being.
The achievement of both goals improves patient adherence to medication, making treatments more successful, while also reducing costs of healthcare, especially for chronic conditions. This is increasingly important, due to changing demographics (aging), and children with medical complex needs. Click here to read about the importance of applying empathy in new drug development, and the challenges faced by patients with swallowing problems.
Matrix tablets are selected to formulate sustained release oral dosage forms because of ease and the economics of their manufacture. These products can be easily manufactured by direct compression or wet granulation depending on the type of polymer and additional excipients used in the formulation.
Generally, active drug substances best suited for incorporation into matrix systems exhibit the following characteristics:
- They exhibit neither very slow nor very fast rates of absorption and elimination. Drugs with slow rates of absorption or elimination half lives in excess of 10 hours are inherently self-retarding.
- Drug substances that have very short half-lives below 2 hours require special care during development because of potential huge fluctuations in plasms levels
- Drug substances whose solubility in aqueous media should be good and also maintain adequate residence time in the gastrointestinal tract.
- Active ingredients that are poorly absorbed or at unpredictable rates are also not good candidates for sustained release formulations.
- Finally, drug substances with very narrow therapeutic windows or really small doses are also poor candidates for sustained release formulations because of the difficulty of limiting control of release and preventing dose dumping.
Types of matrix forming excipients
The different materials currently used to formulate matrix tablets can be classified into four groups on the basis of the basis of their drug retarding mechanism:
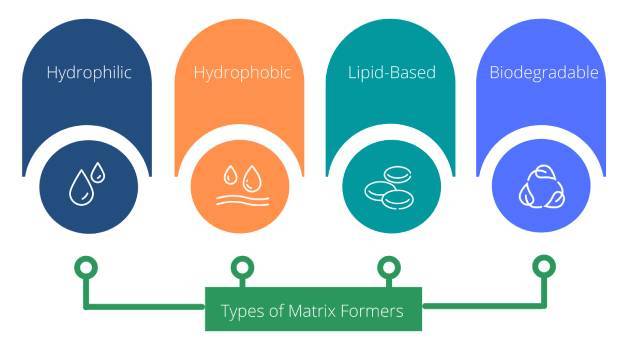
Hydrophobic matrix formers
Hydrophobic matrix materials are water-insoluble large molecular weight polymers for use in the fabrication of sustained release dosage forms. Examples of hydrophobic matrix formers include
- Polyethylene
- Polyvinyl chloride
- Ethylcellulose, and
- Acrylate copolymers
Sustained drug release is produced as a result of swelling and diffusion through a network of channels that exist between compacted polymer particles. The rate-controlling step in these formulations is speed of liquid entry into the matrix.
Lipid matrix formers
Lipid-based matrix formers are high melting point fatty acids and/or waxes capable of forming matrices for sustained release. Drug release from such matrices occurs through both pore diffusion and erosion.
Release characteristics are therefore more sensitive to digestive fluid composition than to purely insoluble polymer matrices.
Examples of lipid matrix formers include
- Carnauba wax in combination with stearic acid,
- Glyceryl dibehenate
- Glyceryl tristearate
- Tripalmitin
- Trymyristin, and
- Hard Fats
Lipid matrices are inert, non-eroding and non-dissolving systems that achieve drug release prolongation through the creation of a hydrophobic domains in the tablet. These domains not only slow down the hydration of the tablet but they also control the rate of dissolution and release of the drug into the aqueous milieu.
Hydrophilic matrix formers
Hydrophilic matrix formers are the most widely used materials of their kind owing to their ease of use, cost-effectiveness and wider regulatory acceptance. They are typically hydrophilic, high molecular weight polymers with high gelling capacities. Upon contact with water, they swell and gel, creating a moving barrier that regulated the rate of drug release. For this reason, they are also known as swelling controlled release systems.
Polymers used in the preparation of hydrophilic matrices are divided in to three broad groups
- Cellulose derivatives, such as methylcellulose, hydroxypropylcellulose, Hypromellose, and Sodium carboxymethylcellulose.
- Non cellulose natural or semi synthetic polymers such as Agar-Agar; Carob gum; Alginates; Xanthan gum, Pectin, Chitosan and Modified starches.
- Polymers of acrylic acid, such as Carbomer 934
Biodegradable matrix
The last category of matric forming materials are a special group of polymers that are biologically degraded or eroded by enzymes to generate simple metabolites that are eliminated through the usual processes. These polymers may be natural polymers such as proteins and polysaccharides, semi-synthetic polymers or fully synthetic systems, such as the well-known aliphatic poly (esters) and poly anhydrides.
Read about the key differences between Hypromellose and Polyethylene Oxide matrix forming excipients here:
Hypromellose v Polyethylene Oxide for the Formulation of Matrix Mini Tablets
Examples of drug products that use matrix former technology
Here are examples of tablets formulated as matrix systems for controlled release:
Theophylline
Theophylline is a methylxanthine, chemically similar to caffeine. It is one of the drugs used in the treatment of asthma and COPD due to its safety and low cost. Theophylline sustained release tablets use hydrophilic polymers such as hydroxyethylcellulose and hypromellose in combination with polyethylene glycol 6000 and cetostearyl alcohol (as a dissolution rate retardant).
Paliperidone
Paliperidone, which is also referred to as 9-hydroxyrisperidone, is the primary active metabolite of risperidone. It is a widely prescribed antipsychotic medication today. The oral dosage forms are available as sustained release tablets using polyethylene oxide and hydroxyethylecellulose as the matrix forming excipients.
Oxycodone
Oxycodone is a semisynthetic opioid drug substance derived from thebaine. It is similar to hydrocodone and morphine. It is currently used for moderate to severe pain due to its powerful analgesic and sedative properties. It exhibits excellent oral bioavailability and is commonly used and/or co-formulated with other analgesics. It is available as sustained release tablets, immediate release tablets, oral solutions and injectables. Matrix tablets when used as the drug release prolonging technology make use of ammoniomethacrylate copolymer (Eudragit® RS 30D), polyvinyl acetate and Glyceryl dibehenate.
Felodipine
Felodipine is a methyl and ethyl diester of 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid. Together with amlodipine and nifedipine, felodipine is a calcium-channel blocker that lowers blood pressure by reducing peripheral vascular resistance through a highly selective action on smooth muscle in arteriolar resistance vessels.
Felodipine sustained release tablets are variously formulated using either hydrophilic or hydrophobic matrix formers, such as Hypromellose K4M and hydrogenated castor oil.
Benefits of using matrix forming excipients
The reasons why particular matrix formers are selected for use by pharmaceutical formulation scientists are many and varied. There is no doubt, however, that as a technology class, these materials offer many advantages, which are outlined below:
- Improve patient convenience and compliance by enabling the formulation of prolonged release dosage forms (e.g once-a-day tablets) that reduce dosing frequency
- Highly versatile in terms of the active ingredients that can be formulated and the manufacturing methods
- Enable the reduction of drug toxicity since the decrease plasma fluctuations and maintain drug within the therapeutic window
- They can enhance product stability
- Prolonged release drugs permit the use of lower amount of total active ingredients thereby reducing drug utilization and exposure
- Improves bioavailability of some drugs
- Matrix formers are relatively inexpensive as a technology
Summary about pharmaceutical matrix formers
Matrix tablets are widely used in the development of sustained release products. A wide range of inactive ingredients known as matrix formers, including hydrophilic, hydrophobic and biodegradable polymers, as well as high melting point lipids are used in matrix tablets. These systems are popular because they are well studied and are amenable for manufacture on standard equipment and processes.
Sources
Pharmacentral has a strict referencing policy and only uses peer-reviewed studies and reputable academic sources. We avoid use of personal anecdotes and opinions to ensure the content we present is accurate and reliable
- Wise, D. L. (2000). Handbook of Pharmaceutical Controlled Release Technology. New York, Marcel Dekker.
- Spiepmann J, Peppas NA (2001). Modelling of Drug Release from Delivery Systems Based on Hydroxypropyl Methylcellulose (HPMC). Adv. Drug Deliv. Rev. 48: 139-157. DOI: 1016/s0169-409x(01)00112-0. Pubchem Google Scholar
- Shargel L, Wu-Pong S, Yu ABC (2012). Applied Biopharmaceutics and Pharmacokinetics, 6th ed., McGraw-Hill Companies.