Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Croscarmellose Sodium is crosslinked Sodium carboxymethylcellulose for use as a superdisintegrant in solid oral formulations (tablets, capsules and granules). It is supplied as a white or greyish-white, odourless, fibrous powder that is insoluble in water.
Pharmacopoeial Compliance: USP-NF; Ph.Eur; JP; IP
Synonyms & Trade Names: Crosslinked Sodium Carboxymethylcellulose; Modified Cellulose Gum, E468; Ac-Di-SoI®; EXPLOCEL®; NYMCEL® ZSX; PHARMACEL® XL; PRIMELLOSE®; SOLUTAB®; VIVASOL®
Uses & Applications: Tablet, Granule, and Capsule Superdisintegrant
Croscarmellose sodium (alternatively known as crosslinked carboxymethylcellulose—sodium salt) is a pharmaceutical excipient and a member of the trinity of well-known superdisintegrants. It is widely used by pharmaceutical R & D professionals for its superior and consistent performance in oral solid dosage forms, compatibility with a wide range of active ingredients, and amenability with direct compression, wet granulation, roller compaction, and capsule filling.
Croscarmellose sodium is supplied as a white or grey-whitish fibrous powder. It is insoluble in water but swells rapidly. Under a microscope, Croscarmellose sodium appears as irregularly-shaped particles, as show below (Magnification x500):
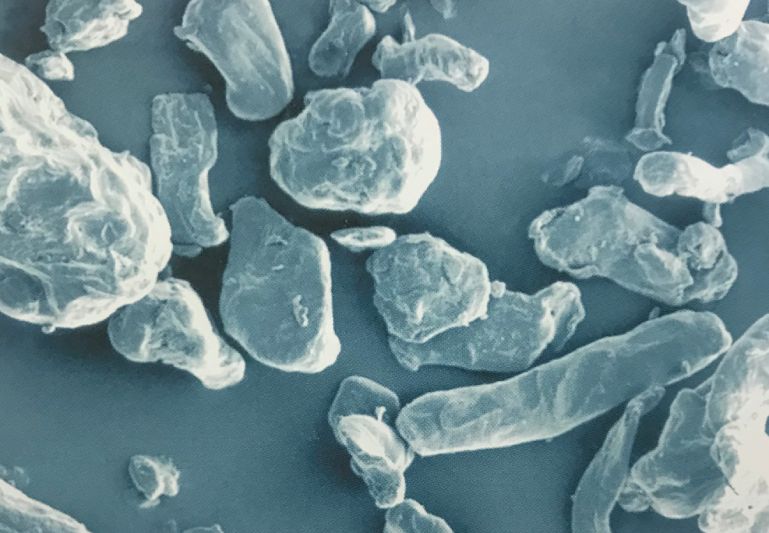
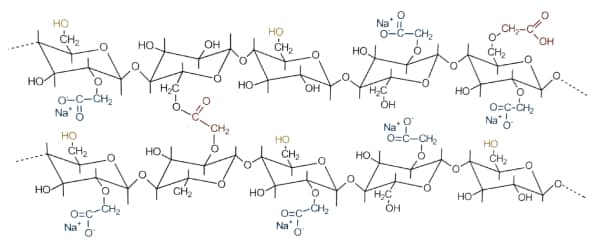
The basic backbone in Croscarmellose sodium is sodium carboxymethylcellulose, a polymer constituted from -D-glucose units but with carboxymethyl groups in place of hydroxy groups on C6. The general chemical formula is shown below.
[C6H7O2(OH)x(OCH2COONa)y)n
where:
Sodium carboxymethylcellulose is obtained by reacting cellulose from specific wood sources with sodium hydroxide, followed by treatment with monochloroacetic acid. The reaction products are purified, and any excess acids are neutralised. Crosslinking is then achieved by reacting the sodium carboxymethylcellulose with acids under heat.

The addition of carboxymethyl groups into the cellulose chemical structure changes the hydrogen bonding in the polymer, opening up the polymer by rendering it highly hydrophilic, absorbent, and water soluble. The addition of crosslinks, however, has the opposite effect of reducing water solubility and viscosity (gelling tendency).
Thus, by optimising the degree of substation and the extent of crosslinking, a grade of crosslinked carboxymethylcellulose sodium can be obtained that has the requisite water uptake and gel formation/solubility.
Interestingly, the Ph. Eur does not specify the degree of substation despite its impact on the material’s properties (the USP – NF does). Commercial products have degrees of substitution that vary from 0.6 to 0.85, with a value of 0.4 being the most optimum.
| Chemical Name | Cellulose, carboxymethyl ether, sodium salt, cross-linked |
| CAS Registry Number | [74811- 65-7] |
| Empirical Formula | C28H30Na8O27 |
| Molecular Weight | 98,000 – 250 000 Daltons |
| EC/ECS Number | 232-674-9 |
| UNII Code (FDA) | M28OL1HH48 |
Croscarmellose Sodium is an approved pharmaceutical excipient currently approved for use in pharmaceutical products all over the world. It is listed in all major pharmacopoeias, including the following:
Croscarmellose sodium is included in the FDA Inactive Ingredients Database (covering oral tablets and capsules). A specification for croscarmellose sodium is included in the Food Chemicals Codex (FCC).
| Physical form | Solid, powder |
| Appearance | White or greyish white powder |
| pH | 5 – 7 |
| Bulk density | 0.400 – 0.600 g/ cm3 |
| Tapped density | O.810 g/cm3 |
| True density | 1.541 g/m3 |
| Particle size | d50 50µm |
| Solubility | Insoluble in water. Swells rapidly 4 – 8 times its original volume on contact with water. Practically insoluble in ethanol |
| Specific surface area | 0.81—0.83 m2/g |
| USP-NF | Ph.Eur | |
| Official name | Croscarmellose Sodium | Croscarmellose Sodium |
| Authorised use | Excipient | Excipient |
| Definition | specified | specified |
| Appearance | White or greyish powder | White or greyish powder |
| Identification | specified | specified |
| pH | 5.0- 7.0 | 5.0- 7.0 |
| Sodium glycollate and Sodium chloride | ≤0.5% | ≤0.5% |
| Water soluble substances | 1 – 10% | 1 – 10% |
| Chlorides | 0.25% | 0.25% |
| Heavy Metals | ≤10ppm | ≤10ppm |
| Loss on Drying | ≤10.0 | ≤10.0 |
| Sulphated Ash | ≤14 – 28% | ≤14 – 28% |
| Settling Volume | 10 – 30 ml | 10 – 30 ml |
| Microbial Contamination | 103 cfu/g | 103 cfu/g |
| Assay | n/a | n/a |
| Labelling | specified | n/a |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Croscarmellose sodium is a pharmaceutical excipient and one of the most frequently used disintegrants in oral pharmaceuticals, both in capsule and tablet formulations. It is also suitable for use in wet granulation, direct compression, roller compaction, and extrusion-spheronisation.
Owing to its effectiveness, Croscarmellose sodium can be used in concentrations as low as 0.25%, although typical concentrations are between 1% and 6% for tablets, with the optimum concentration being around 4%.
Owing to its hydrophilic properties, Croscarmellose sodium imbibes water rapidly and swells significantly (4–8 fold) in under 10 seconds. The action of pulling water into the tablet core reduces the physical bonding forces between particles, which leads to rapid disintegration. This mode of action is illustrated through the infographic below:
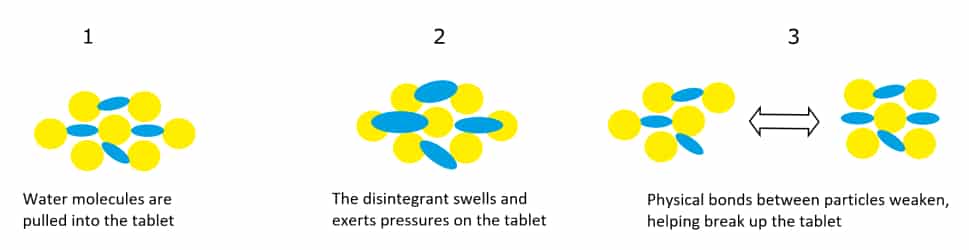
Croscarmellose sodium may be sourced from wood pulp or cotton flock cellulose. The source of cellulose has an impact on several important properties of the material. For instance, wood pulp-derived Croscarmellose sodium tends to have a lower molecular weight, higher solubility, but decreased water binding and swelling capacities when compared with cotton flock-derived material.
Another important property that influences disintegrant properties is particle size. The larger the particle size, the greater the water uptake and, hence, swelling properties. This effect is attributed to the formation of a viscous layer upon contact with water, and a smaller particle size forms a more viscous layer because of enhanced interactions with water.
| Application | Concentration |
|---|---|
| Tablets | 1.0 – 5.0 (typically, 3% for Wet granulation; 2% for direct compression) |
| Hard Capsules | >6.0% |
| Orally Disintegrating Tablets | 3 - 5% |
| Pellets, Granules & Powders | 1-2% |
| Ingredient | % | Amount (mg/tablet) |
|---|---|---|
| Valsartan | 50 | 80 |
| Microcrystalline cellulose | 15.75 | 25.2 |
| Calcium phosphate dihydrate | 31.25 | 50 |
| Croscarmellose sodium | 2 | 3.2 |
| Magnesium stearate | 0.5 | 0.8 |
| Colloidal silicon dioxide anhydrous | 0.5 | 0.8 |
| Total | 100 | 160 |
| Ingredient | % | Amount (mg/tablet) |
|---|---|---|
| Pseudoephedrine HCl | 25 | 60 |
| Calcium phosphate dihydrate | 55 | 132 |
| Microcrystalline cellulose PH101 | 12 | 28.8 |
| Povidone K30 | 4 | 9.6 |
| Croscarmellose sodium | 3 | 7.2 |
| Glyceryl Tristearate | 1 | 2.4 |
| Total | 100 | 240 |
When selecting a grade of Croscarmellose sodium, attention should be paid to its impurity profile. The common impurities in Croscarmellose sodium include chloroacetic acid, nitriles, and nitrates, which are potentially reactive with active ingredients. Furthermore, adsorption of some weakly basic drugs and their salts to Croscarmellose sodium has been reported, and this may lead to incomplete drug release characteristics.
Contrary to what manufacturers claim in promotional literature, Croscarmellose sodium works best with insoluble excipients (such as Dicalcium phosphate and Calcium carbonate) rather than soluble (such as Lactose monohydrate and Mannitol) fillers. Also, the use of alkaline fillers (e.g., sodium carbonate, sodium bicarbonate, and calcium carbonate) is accompanied by a slowdown in dissolution upon storage, with the decrease being proportional to the increase in alkalinity.
Croscarmellose sodium has been used in pharmaceutical and dietary supplement tablets for several decades and has been shown to be a safe and well-tolerated chemical substance. Regulatory authorities consider it a generally non-toxic and non-irritant excipient. It is closely related to another widely used chemical substance, Sodium carboxymethylcellulose, which has been shown to be safe.
The European Food Safety Agency reported findings from a 3-month oral toxicity study in rats that were fed increasing doses of croscarmellose sodium (0, 10,000, and 50,000 ppm). No treatment-related adverse effects or deaths were observed. Furthermore, body weight, food consumption, ophthalmology, haematology, clinical chemistry, gross pathology, and histopathology were not significantly altered, except at the 50,000 ppm dose.
It was concluded that NOAEL (No Observed Adverse Effect Level) was 10,000 ppm, equivalent to 757 mg/kg/day in males and 893 mg/kg/day in females. In a teratogenicity study in rats, dietary administration of up to 50,000 ppm (approximately 4554 mg/kg/day) during days 6–15 of gestation produced no maternal toxicity, embryo-fetotoxicity, or teratogenic effects.
Thus, EFSA’s recommended usage level of Croscarmellose sodium in dietary tablets is NMT 30 mg/kg/day. The World Health Organisation has not specified an ADI for Croscarmellose sodium when used as a food additive since the levels required to achieve the disintegrant properties are lower than what would be required to cause health hazards.
Handling Croscarmellose sodium in an industrial setting can represent a dust hazard. To avoid irritation of the eyes or nasal passages, adequate safety precautions should be taken.
Although hygroscopic, Croscarmellose sodium is a stable excipient when properly stored under ambient conditions. In tablet formulations, there is no discernible impact on disintegration and dissolution when croscarmellose sodium is used as a superdisintegrant followed by storage at 30 °C for a year. That said, the bulk material should be stored in a closed container away from direct light or moisture for maximum longevity.
Croscarmellose sodium is sourced from cellulose, and it is 100% biodegradable. It does not accumulate in the environment and has no long-term impact on ecology or marine life. Croscarmellose sodium excipient grade achieved a total score of 78/100 by the Excipients Forum Sustainable Chemistry™ Score.
[1]. D.S. Bindra, D. Stein, P. Pandey, N. Barbour, Incompatibility of croscarmellose sodium with alkaline excipients in a tablet formulation, Pharm Dev Technol, 19 (2014) 285-289. DOI: 10.3109/10837450.2013.778869. PUBMED
[2]. R. Eyjolfsson, Tablets with high lactose content: effects of some binders and disintegrants on their properties, Pharmazie, 60 (2005) 711-712. PUBMED
[3]. K.A. Khan, C.T. Rhodes, Water-sorption properties of tablet disintegrants, J Pharm Sci, 64 (1975) 447-451. DOI: 10.1002/jps.2600640321. PUBMED
[4]. B.R. Rohrs, T.J. Thamann, P. Gao, D.J. Stelzer, M.S. Bergren, R.S. Chao, Tablet dissolution affected by a moisture mediated solid-state interaction between drug and disintegrant, Pharm Res, 16 (1999) 1850-1856. DOI: 10.1208/pt060119 PUBMED
[5]. H.V. van Kamp, G.K. Bolhuis, C.F. Lerk, Improvement by super disintegrants of the properties of tablets containing lactose, prepared by wet granulation, Pharmaceutisch weekblad. Scientific edition, 5 (1983) 165-171. DOI: 10.1007/BF01961475. PUBMED
[6]. N. Zhao, L.L. Augsburger, The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of hydrochlorothiazide from directly compressed tablets, AAPS PharmSciTech, 6 (2005) E120-126. DOI: 10.1208/pt060119 PUBMED
[7]. F. N. Munoz, M.V. Velasco, A. Muñoz-Ruiz, R. Jiménez-Castellanos, Disintegrating efficiency of croscarmellose sodium in a direct compression formulation, International Journal of Pharmaceutics, 147 (1997) 11-21. DOI.org/10.1016/S0378-5173(96)04784-9
[8]. The U.S.P. Convention, Food Chemicals Codex (11th Edition), (2018).
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.