Drug discovery and development can be described as the sum total of steps taken by research-intensive entity to identify a new chemical or biological substance and transform it into a product approved for use by patients.
This highly knowledge and capital intensive process takes, on average, 10-15 years and over $2 billion (2021 figures), to pull off.
But exactly what is involved? Who does what and when? In this article, we outline key concepts of drug discovery and development, including target identification, clinical trials, pharmaceutical development and commercialisation.
Drug Development
Drug development covers all the activities undertaken to transform the compound obtained during drug discovery into a product that is approved for launch into the market by regulatory agencies. This is a pivotal process, and a lot rides on its success, thus, efficiency is absolutely critical, but mainly for two key points:
Firstly, development is expensive – accounting for 70% of the total R&D costs. Even though the number of projects is much smaller compared with those under discovery, the cost per project is significant, and increases exponentially as the project progresses through into the latter stages.
Secondly, speed is the essence in drug development as it determines how soon the company can start earning a return on the huge investment ploughed into the project. Besides, any delays eat away into exclusivity arrangements granted by patents and time before generic manufacturers can launch me-too products.
For clarity, drug development is presented as an operation that’s distinct from discovery. In reality, the distinction is not as clear cut. Often, different activities are being undertaken concurrently, and in deed, some processes that were traditionally undertaken much later on are increasingly being brought much forward. The idea is to identify compounds that have the highest chances of success much earlier and focus on those.
Components of drug development
The key activities involved in the development of a typical are summarised in the diagram below, showing the different tasks that are undertaken during this process. Generally, these tasks can be divided into three parts: technical; investigative and administrative.
General Perspective – New Drug Discovery and Development
The process of creating a new drug product can be broadly divided into three main phases:
- Drug discovery – entailing the conceptualisation of the therapeutic into a molecule with known pharmacologic effects
- Drug development – covering the steps taken to convert the molecule above into an approved and registered drug product
- Commercialisation – which includes all the steps taken to convert the product into an approved therapeutic, launch into the market and to generate sales
These processes are schematically illustrated below, which is greatly oversimplified:
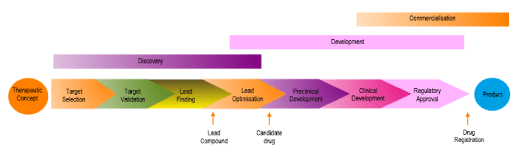
Historically, these functions were performed, respectively by the Research, Development and Marketing departments. Nowadays though, a number of these functions are outsourced to other companies that specialise in one or more aspects of these activities.
It’s worth emphasising that many activities described in the above scheme may proceed in parallel and other may spill out into other phases. For example, development activities, such as clinical trials or additional testing of formulations, generally continue well beyond registration of the drug product. Such tests may be driven by the requirement for more understanding about the new drug or the need to extend use beyond the main therapeutic applications.
The job of the discovery teams does not end with product registration and market launch. Many discovery scientists will carry out more research looking for other candidates to serves as backups in case the lead compound fails or as follow-on compounds that might have better safety or efficacy profiles over the lead compounds.
Finally, the three processes listed in the new drug development scheme above are not independent and consecutive. Rather, they are coordinated with each other because the performance of each process influences decisions taken in another stage.
Understandably, there are many competing interests as the new drug progresses through its journey. To successfully fit in and integrate all the different interests and cultures, effective project management skills are required.
Therapeutic Concept Selection
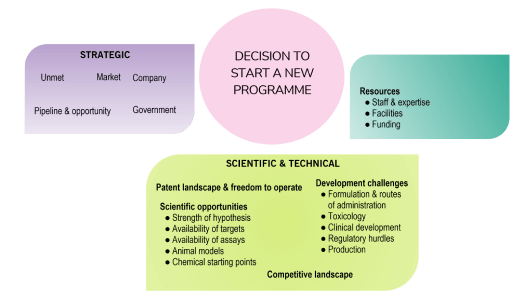
Therapeutic concept selection is about deciding whether or not to embark on a new 0project. Success at this stage is measured in terms of agreeing and signed off a drug discovery program with a clear-cut aim and timeline.
Exactly where the idea originates varies from company to company. Some companies have very strong exploratory research teams that undertake research internally and discover new knowledge on diseases and druggable targets. Others are more open minded, preferring to purchase new molecules in for further development. Often, it’s a mixture of both approaches.
Generally, the decision to select a particular program will be guided by three things: company strategy, technical capabilities and operational constraints.
- Should the company do it? (Strategy)
- Could the company do it? )scientific and technical capabilities)
- Can the company deliver it? (operational constraints)
These three factors are summarised below:
Drug Discovery
The drug discovery process technically starts with choice of a disease area and a definition of the therapeutic need that should be addressed. Once this is done, the process proceeds to identification of the physiological mechanisms that need to be targeted, and ideally, identification of a specific molecular ‘drug target’.
During this phase, effort is focussed on identifying a lead chemical structure, designing, testing and fine-tuning it and ensure that it meets all the criteria required for development into a drug product.
An overview of the main stages that constitute a typical drug discovery project, from the point of identification of a target to the production of a candidate drug is shown below this process is shown below:
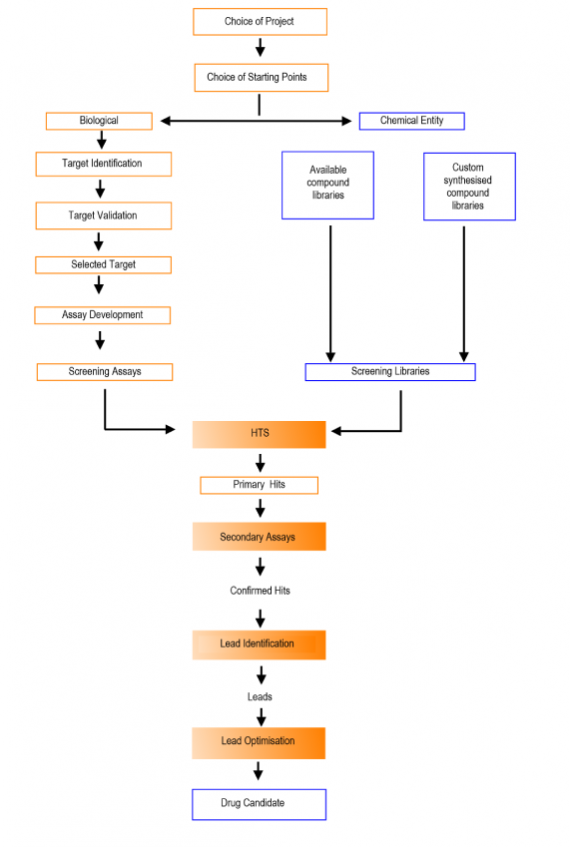
Discovery can at first appear like a shot in the dark. At the start, scientists will be dealing with a huge number of compounds (1020), which have to be filtered, mainly via computer simulations (in silico) into manageable number capable of being further optimised.
High throughput screening (HTS) is then applied to identify ‘HITS’ which demonstrate interesting activity. Since HTS can throw up a huge number of ‘HITS’ these are further optimised and validated to remove any artefacts or ‘noise’ from the screen.
A key aspect of validation stage is to find relationships between chemical structure and biological activity, and to find out if the compounds belong to any existing families of compounds (known as hits series).
Validated hits are therefore further studied, especially in terms of their pharmacokinetic profiles and toxicity. At this point, the number of compounds has reduced to a handful. It is these handful of substances that are subsequently entered into the lead optimisation programme.
Lead optimization is a critical process in drug discovery since it’s determines whether a suitable compound can be identified for taking forward into preclinical and clinical studies. Therefore, the goal of this stage is to scrutinise and fine-tune, typically in parallel, both the biological activity and the physicochemical properties of the lead series.
During this stage, rigorous data is generated in a precise, timely manner to quickly determine the compounds to progress the compounds, and the series, toward the ideal candidate profile. The higher the quality of these candidates, the higher the chances of successful progression into clinical trials.
“MAGIC BULLETS”
The term ‘magic bullet’ was coined by Paul Ehrlich (1854 – 1915), a Germany medical scientist and winner of the Nobel Prize in Physiology or Medicine in 1908. Ehrlich envisaged a compound (the bullet) capable of attacking a pathogen and destroying it while leaving its host intact. Nowadays, pharmaceutical scientists are developing targeted and personalised cancer therapies, and these, many argue, are modern realisations of Ehrlich’s idea.
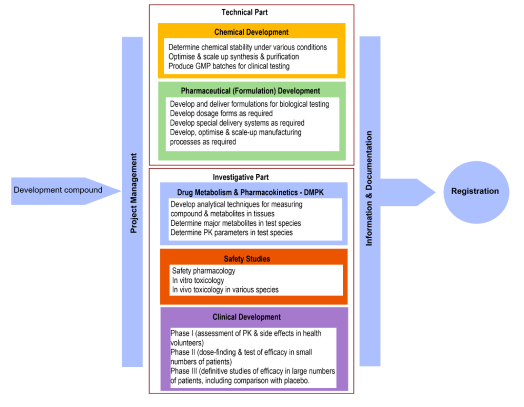
Technical development – solving technical issues related to synthesis and formulation of the drug substance with the aim of ensuring the quality and safety of the drug product. The key functions involved here are chemical, manufacturing and formulation development.
Investigative studies – establishing the safety and efficacy of the product, including assessment of whether it’s pharmacokinetically suitable for clinical use. The main function involved here are safety pharmacology, toxicology and clinical development.
Administrative functions – coordinating and managing quality control, logistics, communications and decision making to ensure high quality data are generated and to minimise any delays. The main function involved here is project management.
In addition, there will be a team coordinating and liaising with regulatory authories, collating data, liaising with material suppliers and writing dossiers for presentation to authorities in order to gain approval in a timely manner.
Pharmaceutical Development
This stage is also known as pharmaceutical development. Since pure drug substances are rarely suited for clinic use, they need to be formulated; by combining them with excipients, into tablets, capsules, injections, etc.
Pharmaceutical development refers to all the different tasks undertaken to transform the drug substance identified as a candidate during the discovery phase into a dosage form that is able to reliably deliver the drug into the body in a safe and reliable way.
Designing a formulation can be as equally time consuming and complex as drug discovery. In order to mitigate some of the issues that crop up, initial studies are undertaken during lead optimisation, before development actually starts.
For conventional drug substances, the desired route of administration is the oral route. Alternatives are considered, particularly if sufficient bioavailability cannot be achieved orally.

The different tasks undertaken in pharmaceutical development can be grouped into two:
- Preformulation studies which specifically investigate physical and chemical properties of the drug substance, such as solubility and dissolution rates; acidity and alkalinity (pKa), chemical and physical stability, lipophilicity, particle morphology, melting point and fracture behaviour, etc.
- Formulation studies which are essentially, chemical engineering effort aimed at converting a powder or a liquid form of the drug substance into a stable and deliverable product. During this process, formulation scientists will take into account all the known properties of the drug substance and the desired drug delivery system that best meets the therapeutic objectives of the compound.
Clinical Development
Clinical development is an umbrella terms to describe the whole set of activities undertaken by the team in support of testing of a new drug substance in humans. It includes the following clinical trials, which refer to administration into man the new drug under controlled conditions to investigate bioavailability, efficacy, safety, tolerability and acceptability.
Clinical development of new drugs has been described as both a science and an art, since it requires technical expertise, sound judgement and commercial acumen.
Executed well, clinical development brings new medicines quickly and safely to patients who need them, while also managing to return on the financial investment.
At the time of writing this post In 2021, estimates of the investment required for clinical development studies vary, but most sources agree that, including the cost of failures and capital invested, the total cost of bringing a new drug to market is $2-3 billion, spread over a 12-year development cycle. Out of this cost, two-thirds is spent on clinical development.
The clinical development process is divided into four phases, summarised in the table below.
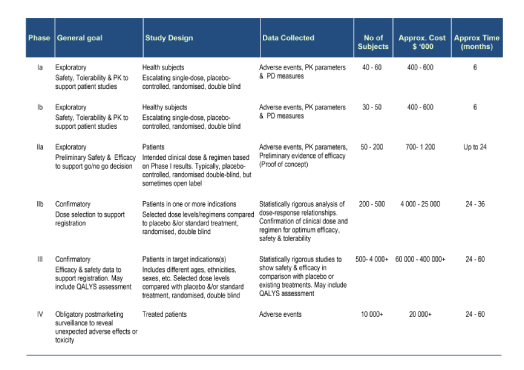
- Phase I: first introduction and safety assessment in man, typically in healthy volunteers
- Phase II: early exploratory and dose-finding studies in patients
- Phase III: large scale studies in patients
- Phase IV: post-marketing safety monitoring of patients.
In almost every country on earth today, clinical trials are a legal requirement before a new drug can be sold or claims made for its safety of benefits. This does not include alternative remedies, however. In addition, all clinical trials, include Phase I studies, are subject to international, national and in most cases, institutional regulations.
The different international regulations and requirements are set out in guidelines published by the International Conference on Harmonisation (ICH), an organisation that was set up to harmonise pharmaceutical regulations across Europe, United States and Japan.
It is a requirement that clinical development procedures be done under ICH guidelines if the results are to be accepted for registration in the three ICH guidelines. This does not mean that the studies need to be done there – they only have to comply with these guidelines.
In addition to ICH guidelines, human studies are required to be undertaken according to an ethical framework defined code, known as the The Declaration of Helsinki (2000). This code, in a nutshell, requires the principal investigator (physician) to protect life, health, privacy and dignity.
Regulatory Affairs
A fundamental maxim in pharmaceutical new product development is the basic division of responsibilities whereby the health authority, such as the US FDA, is responsible for safeguarding the public’s health against defective and unsafe products, and the pharmaceutical company being responsible for all aspects of drug product development (quality, safety and efficacy).
The regulatory authority develops regulations and guidelines for companies and others in the value chain to follow. The approval of a pharmaceutical product is a contract between the regulatory authority on behalf of the public, and the pharmaceutical company.
The regulatory authority is responsible for approving clinical trial applications, approving marketing authorisation applications, and monitoring safety and efficacy claims of the marketed drugs. Authorities can withdraw the approval at any point where there are cases of non-compliance.
The conditions of the approval are set out in a dossier and appear in the prescribing information. Changes to these terms have to be pre-approved and authorised before they can be implemented.
Within pharmaceutical companies, the regulatory affairs department is the one responsible for obtaining approval for the new pharmaceutical product and working to ensure that this approval is maintained for as long as the company desires so.
Regulatory affairs professionals work at the interface between the regulatory authority and the project team, and they’re often the channel of communication between external and internal stake holders with respect project’s regulatory standing and progress.
Milestones and Decision Points
The decision to advance a drug candidate into early development is the first of several key strategic decision points in a new drug development project. The timing, naming and decision-making process vary from company to company, the one conceptualised below was developed by Norvatis:
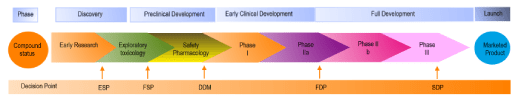
Early selection point (ESP
Is the decision to take the drug candidate molecule into early (preclinical) development.
Decision to develop in man (DDM)
Is the decision to enter the compound into Phase 1, based on information obtained during preclinical development phase. Once this decision is made, the company will aim to produce between 2 and 10kg of clinical grade material.
Full development decision point (FDP)
This point is reached after Phase I and Phase IIa studies have been completed. At this point, the company has some preliminary evidence about clinical efficacy in man. From here on, the project costs skyrocket and the company must be confident on commercial returns.
Submission decision point (SDP)
This is the final point when a decision is taken to apply for registration, based on the data collected and its quality.
Summary Points about Drug Discovery and Development
Pharmaceutical companies undertake research for commercial reasons and their overarching objective is a return on capital invested. This is not to say a few companies include altruistic motives, the fact of the matter is that it takes less priority.
For this reason, the research a company pursues has to be in line with its commercial goals. Curiosity-driven research is generally left to Universities and other institutes. That said, there are territories where the two universes overlap, namely, applied research. Many recent innovations in medicine, such as monoclonal antibodies, fall into this domain, and both pharmaceutical companies, and universities have contributed to their development.
Finally, it should be stated that drug discovery and development is unlike any other type of development or innovation process, such as developing a new car. Drug discovery and development carries far greater uncertainty, and the outcome is rarely assured.
Resources Used
Pharmacentral has a strict referencing policy and only uses peer-reviewed studies and reputable academic sources. We avoid use of personal anecdotes and opinions to ensure the content we present is accurate and reliable
- R.G. Hill, H.P. Rang, Preface to 2nd Edition, in: R.G. Hill, H.P. Rang (Eds.) Drug Discovery and Development (Second Edition), Churchill Livingstone 2013
- Orloff et a.,l The future of drug development: advancing clinical trial design. Nat Rev Drug Discov8, 949–957 (2009). https://doi.org/10.1038/nrd3025