Abstract
In this study, a novel ‘mini tablet’ matrix system was developed using hypromellose and polyethylene oxide to determine if it resolved the commonly faced issue of dose dumping in monolithic systems. Using theophylline as the model drug, dissolution profiles of the matrix mini tablets were obtained with the aid of a USP apparatus 1 and 4 The results indicate two-compartment mini-tablets exhibited slower drug-release as well as an absence of the initial rapid drug-release seen in one-compartment systems. Of the polymer combinations tested, polyethylene oxide (as the internal polymer) and hypromellose (as the external polymer) combination showed the highest potential for resisting dose-dumping when agitation was increased (showing only an increase of 4.1% in drug-release, in comparison to a 42.5% increase shown by a commercially theophylline product, Uniphyllin®), This study shows that the concept of a matrix mini tablet system is a promising option for preventing dose-dumping although further work is required to optimise the system.
Background to the study
The need for frequent oral administration in cases of chronic disease can deter patients’ compliance to medication. While prolonged drug-release formulations can reduce dosing frequency and support better patient compliance and outcomes (Augsburger and Hoag, 2008), the incorporation of large drug concentrations in a single dosage form poses a potential risk to patient’s a health should drug-release characteristics of the dosage form become affected, for instance, when the phenomenon of ‘dose-dumping’ occurs (Mayer and Hussain, 2005).
Dose dumping may be induced by several factors; the commonest ones being consumption of meals before ingestion of medication (Hendeles et al., 1985; Karim et al., 1985, Schmidt and Dalhoff, 2002). Hendeles et al.(1985) found that consumption of a breakfast of bacon and eggs prior to administration of theophylline sustained-release formulations induced dose-dumping. Dose dumping was attributed to exposure to the alkaline bile salts or pancreatic enzymes following postprandial gastric emptying.
Hydrophilic diffusion-based monolithic matrix systems are a popular sustained-release system due to their enhanced reliability, relatively cheap manufacturing cost and ease of formulation (Augsburger and Hoag, 2008). The drug is dispersed evenly in a polymer and upon contact with GI tract fluids, a gel-layer forms to serve as a barrier to fluid movement into and drug movement out of the system. Commonly used polymers for hydrophilic matrices include hypromellose, polyethylene oxide and methacrylic acid copolymers (Tiwari and Rajabi-Siahboomi, 2008, Appicella et al., 1993).
A flaw in the design of monolithic matrix systems is that when conditions promoting rapid drug-dissolution are encountered in vivo, a high proportion of the drug is immediately released into the gastrointestinal (GI) tract, potentially exposing the individual to a high dose (Hendeles et al., 1985). Previous attempts at modulating this initial burst of drug-release have been unsuccessful (Samuelov et al., 1979; Cobby, 1974; Conte (1993).
Novel ‘mini-tablet’ matrix systems, consisting of two-compartments, have been developed in an attempt to modulate drug release and to resolve the consequences of tablet rupture (De Brabander et al., 2000; Efentakis et al., 2000; Lopes et al., 2007). In this context, the two compartments are the mini-matrix tablet domain (internal) surrounded by a continuous polymer formulation (external). The containment of the drug within these internal polymer domains leads to an additional barrier separating the drug from GI fluid, thus preventing the immediate dumping of large quantities of drug. This is shown in figure 1 below.
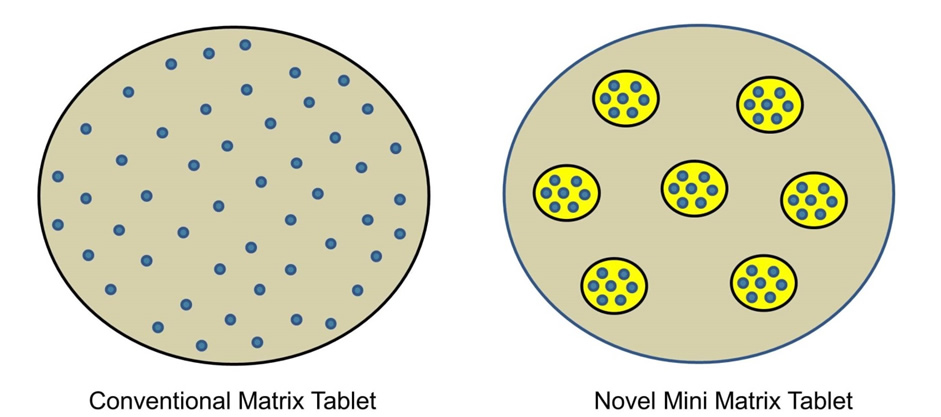 Figure 1: Conceptualisation of the mini-tablet system. Grey area is the external polymer domain; the yellow area is the internal domain polymer and the blue dots represent the drug
Figure 1: Conceptualisation of the mini-tablet system. Grey area is the external polymer domain; the yellow area is the internal domain polymer and the blue dots represent the drug
Objectives of the study
The objectives of the study were to determine the dissolution profiles of four different combinations of theophylline mini-tablets and to assess the suitability of PEO and HPMC as polymers for the internal and external domains of mini-tablets. Drug release profiles were investigated with the aid of the USP Apparatus 1 and 4 were used, with Hanks buffer at pH 7.4 used as the dissolution media. The vessels containing the media were sealed when possible throughout the investigation to prevent the escape of CO2.
Study Methodology
Materials
Theophylline anhydrous was purchased from Sigma Aldrich, UK, Hypromellose (Methocel™ K100M and Polyethylene Oxide (Polyox ™ WSR 1105) were kindly donated by Colorcon UK, Lactose anhydrous was gifted by Kerry Group, Dublin, Ireland, Colloidal Silica (HDK® N20 Pharma) was kindly provided by Wacker AG and Microcystalline Cellulose was purchased from Sigma ALdrich UK. The rest of the materials were technical grade materials purchased from a variety of vendors.
Methods
Preparation of Matrix ‘Mini Tablets’
Mini matrix tablets (total batch size of 400g) were prepared according to the scheme in table 1 and formulas in table 2 below.
Table 1:- The four combinations of the mini-tablet formulations evaluated
| Internal mini-tablet polymer | External polymer | Tags |
| PEO | HPMC | P/H |
| PEO | PEO | P/P |
| HPMC | Hypromellose | H/H |
| HPMC | PEO | H/P |
Table 2: Formulations used to produce mini-tablets
| Ingredient | % (w/W | Quantity g (for 400g) |
| Theopylline | 20.0 | 80 |
| PEO/Hypromellose | 20.0 | 80 |
| MCC | 15.0 | 60 |
| Lactose | 44.3 | 177.2 |
| 7-hydroxycoumarin | 0.1 | 0.4 |
| Magnesium Stearate | 0.5 | 2 |
| Colloidal silica | 0.1 | 0.4 |
In each case, hypromellose or polyethylene oxide was blended with theophylline, microcrystalline cellulose and lactose for 5minutes. Magnesium stearate was then added and blending continued for a further 3 minutes. Finally, the 7-hydroxycumarin and colloidal silica were added and blending undertaken for a further 3 minutes.
All powder blends were assessed for flowability (Carr’s compressibility index and angle of repose). Tablet compression was undertaken on Riva Minipress Single Punch tablet press using 2 mm tooling. The obtained mini matrix tablets were then manually dosed into a 15 mm dies to which a portion of the appropriate external polymer blend had been added and compressed to create the mini matrix tablet system. Each system was designed to contain 50mg of theophylline.
Friability, hardness testing, and uniformity of weight of the macro tablets were performed using an ErwekaTA-120 friabilator and Erweka TBH-220D hardness machine.
Dissolution testing
USP Apparatus 1 dissolution testing at 100rpm
An Erweka DT 600A USP Apparatus 1 machine set to either 100rpm or 150 rpm (bath temperature 37°C) was used. 1L of Hanks buffer (pH 7.4) was placed in each vessel. At intervals of 0.5, 1, 2, 3, 4, 5, 6 and 26 hours, samples were withdrawn and absorbances taken using an Agilent UV spectrophotometer set at 268nm. The withdrawn aliquots were replaced with an equal amount of fresh dissolution media at the same temperature.
USP Apparatus 4: dissolution testing
A Sotax CE7 Smart USP Apparatus 4 was set to a temperature of 37°C and flow rate of 8 ml/min. One 5mm ruby bead was placed at the bottom of each of the 7 Dissotest cells and covered with 1mm glass beads up to one third of the cell. The tablets were placed into cells and Hanks buffer (pH 7.4) used as the test media. Readings were automatically taken at 30minute intervals for 15 hours. Measurements were performed in triplicate.
Results
Mini Tablets Characteristics
Lower compressibility and angle of repose values were obtained for the PEO powder compared with hypromellose suggesting that the PEO powder had better flow and tableting properties. Both powders produced tablets which had the weight and friability values consistent with current British Pharmacopoeia requirements (see table 3).
Table 3: Test results on macro tablets used to make the micro-domains
| PEO | Hypromellose | |
| Powder flowability | ||
| Compressibility (%) | 16.0 % | 24.0 % |
| Angle of repose | 36° | 43° |
| Weight: | ||
| Mean | 0.346g | 0.349g |
| Standard deviation | 0.002006 | 0.002102 |
| Friability (%) | 0.186 % | 0.178% |
| Hardness: | ||
| Mean | 96.1 ± 0.3 N | 88.5 ± 0.4 N |
Dissolution Tests
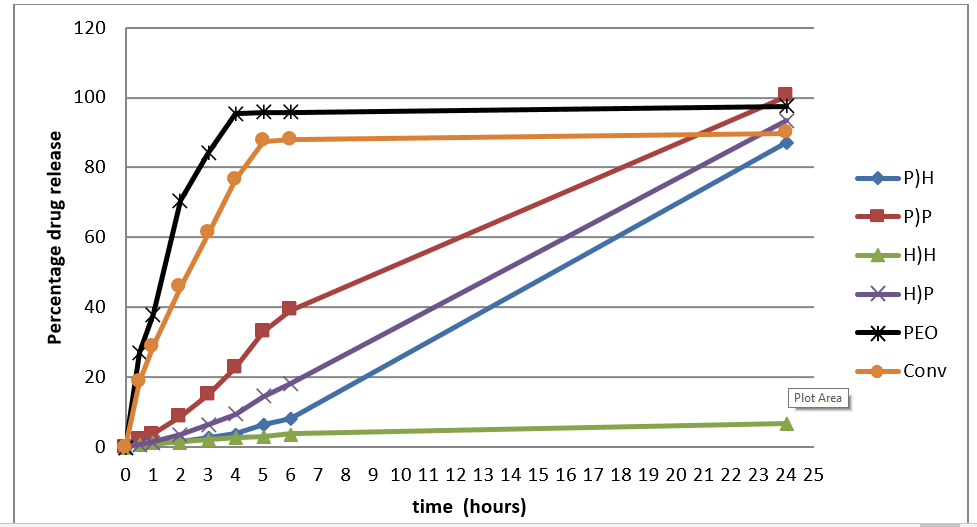
Drug release profile of the matrix systems are shown in Figure 2 to 4. Figure 2 and 3, which pertain to USP Apparatus 1 tests reveal faster drug-release in monolithic matrix tablets compared with the the two-compartment systems. The former also exhibit an initial rapid release of drug within the first 30 minutes. Increasing agitation (100 to 150 rpm) resulted in increase in drug-release. Systems formulated with PEO alone showed the fastest overall drug release profile. This was followed by commercial theophylline product.
The patterns of drug release for mini matrix tablet systems were clearly different from monolithic tablets. Moreover, there were differences between the different polymers with respect to what was added to the external versus internal phases. Finally, the two-compartment systems achieved more prolonged release and also avoided the initial spike in release, a result that is in agreement with what O’Connor (2011) reported.
Figure 2: Dissolution profiles of the 6 dosage forms using USP apparatus 1 at 100rpm
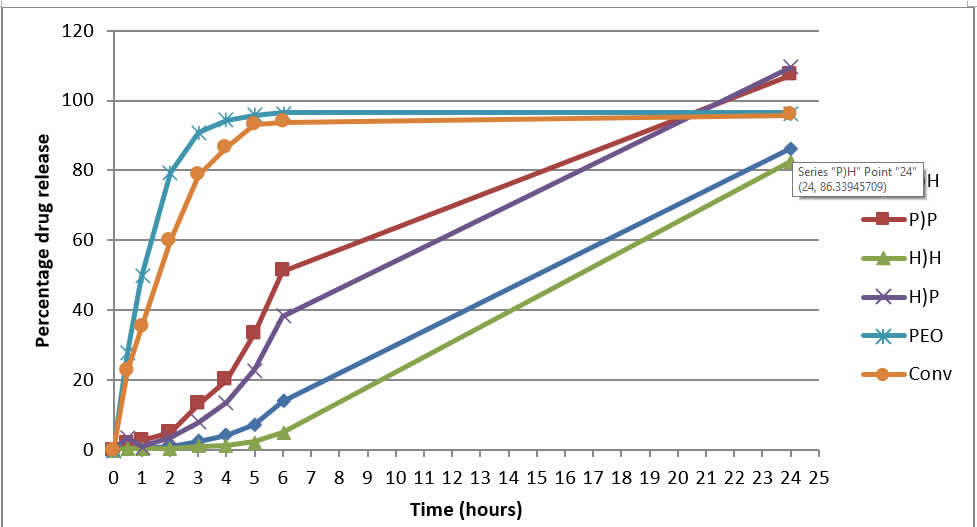 Figure 3: Dissolution profiles of the 6 dosage forms using USP apparatus 1 at 150rpm
Figure 3: Dissolution profiles of the 6 dosage forms using USP apparatus 1 at 150rpm
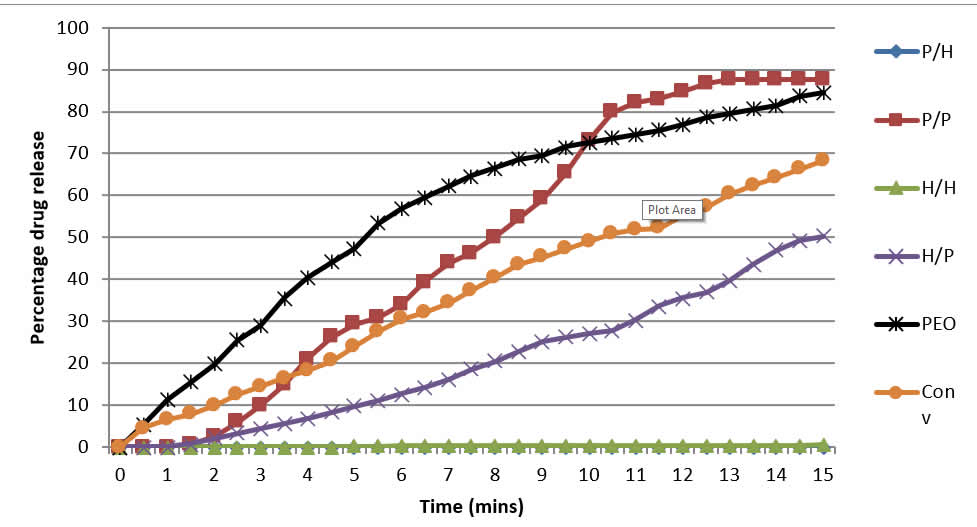 Figure 4 shows the drug release profiles of the matrix systems as obtained from the USP Apparatus 4. The two-compartment matrix systems achieved more prolonged release while avoiding the initial spike in release. The PEO macro tablet had the fastest drug-release rate, followed initially by the commercial tablet. H/P, H/H and P/H systems, too, showed readings only from 1.5 hours. However P/H and H/H did not appear to release drug loads.
Figure 4 shows the drug release profiles of the matrix systems as obtained from the USP Apparatus 4. The two-compartment matrix systems achieved more prolonged release while avoiding the initial spike in release. The PEO macro tablet had the fastest drug-release rate, followed initially by the commercial tablet. H/P, H/H and P/H systems, too, showed readings only from 1.5 hours. However P/H and H/H did not appear to release drug loads.
Figure 4: Cumulative percentage drug-release of 4 dosage forms using USP Apparatus 4
Discussion
The absence of the initial spike in drug-release with minitablets may be due to the lack of drug closer to dosage-form surface as the drug is contained within the internal domains. The distance presents a longer path for both the media and the drug travel, which slows down the diffusion and release of the drug substance. In monolithic matrices, if conditions present that promote drug to be released, there is an increased likelihood of reaching toxic-levels in these systems.
A direct comparison between P/P and the macro tablets highlight the difference in drug-release between one- and two-compartment models. Both used identical polymers yet had very different release profiles. The macro tablet released 50% of its dose in just 1.4 hours (at 100rpm) and 1.0 hours (at 150rpm) compared to the 9.5 hours (100rpm) and 5.9 hours (150rpm) of P/P, respectively representing a 7- and 6-fold increase in the time taken for the two-compartment model.
USP 4 data suggest the drug release characteristics of P/P system are unsuitable due to the rapid rate of drug release, which is even faster than the commercial tablets. This suggests that P/P systems are more prone to dose-dumping. H/H and P/H are also unsuitable as drug-release was extremely low and hence impractical for reaching the required therapeutic drug levels.
Effect of increasing agitation
The robustness of the formulations appears unrelated to whether the dosage forms were of a one- or two-compartment design. The greatest effect on drug release when rotation in USP 1 was increased from 100rpm to 150 rpm was seen on P/P where it showed to be 61% faster for the first 50% release of drug. H/P showed the second largest increase, followed by the commercial tablet at 42.5%, the macro tablet at 35.6%, and P/H at 4.1%. These results suggest presence of the internal domains alone is not enough to assume higher resistance to dose-dumping. Properties of the polymers within the dosage-form must also be considered. An investigation by Maggi et al. (2000) found the PEO gel-layer to be weaker and less-effective in preventing water-entry than the hypromellose gel-layer, resulting in an increased rate of gel-erosion and drug-release.
Figure 5 shows the result of an experiment where pure PEO and HPMC tablets were submerged in water. The presence of undissolved solids at 20 hours within the HPMC tablet system is indicative of hypromellose higher resistance to water entry into the tablet compared to PEO which showed no signs of undissolved polymer from 15hrs onwards. Additionally the greater diameter of the hypromellose tablet after 20 hours indicates the higher strength of its gel-layer.
Figure 5: Dissolution profile of tablets made of pure PEO (left) and HPMC (right) at 8hrs (top) 15hrs (middle) and 20hrs (bottom) (from from Maggi et al., 2000)
Effect of internal and external domains on drug-release
H/P and P/H despite containing both hypromellose and PEO displayed very different drug-release and stability properties. This suggests that the influence of the polymer in the internal domain versus the external domain on drug-release is unequal. Performance at 100rpm of the two systems was selectively compared with P/P. H/P drug-release was only 24.6% less than P/P while P/H drug-release was 56.9% less, more effectively expressing the slower drug-releasing properties of HPMC. This suggests the polymer in the external domain influences drug-release more than the internal domain in two-compartment systems. These results coincide with observations made by Maggi et al. (2000) who found the external layers play the major role in controlling drug release.
Conclusions
PEO and Hypromellose, whether alone or in combination cannot achieve a suitably strong dosage form with good drug-release properties. P/H was the only formulation to show similar drug-release profiles for 100rpm and 150rpm. This may be an indication that this formulation is robust and is unlikely to dose dump. Further optimization is suggested, including the use of lower viscosity grades of hypromellose in the P/H formulation to tailor drug-release while maintaining resistance to erosion. Varying the external polymer as the internal domains appears to have less effect on the drug-release properties of the dosage-form and so should be kept constant.
References
Apicella, A. Cappello, B. Del Nobile, M. La Rotonda, M. Mensitieri, G. Nicholais, L. (1993). Poly (ethylene oxide) (PEO) and different molecular weight PEO blends monolithic devices for drug release. Biomaterials. 14 (2), 83-90.
Augsburger, L. and Hoag, S., 2008. Pharmaceutical Dosage Forms: Tablets. 3rd ed. Vol. 2: Rational Design and Formulation. Informa Healthcare USA, Inc.
De Brabander C, Vervaet C, Görtz JP, Remon JP, Berlo JA. Bioavailability of ibuprofen from matrix mini-tablets based on a mixture of starch and microcrystalline wax. Int J Pharm. 2000 Nov 4;208(1-2):81-6. doi: 10.1016/s0378-5173(00)00549-4. PMID: 11203270.
Cobby, J. Mayersohn, M. Walker, G. (1974). Influence of Shape Factors on Kinetics of Drug Release from Matrix Tablets. Journal of Pharmaceutical Sciences. 63 (5), 732-737.
Conte, U. Maggi, L. Colombo, P. La Manna, A. (1993). Multi-layered hydrophilic matrices as constant release devices ( GeomatrixTM Systems*). Journal of Controlled Release. 26, 39-47.
Efentakis M, Koutlis A, Vlachou M. Development and evaluation of oral multiple-unit and single-unit hydrophilic controlled-release systems. AAPS PharmSciTech. 2000;1(4):E34. Published 2000 Dec 1. doi:10.1208/pt010434
Hendeles, L. Weinberger, M. Milavetz, G. Hill, M. Vaughan, L. (1985). Food-induced “dose-dumping” from a once-a-day theophylline product as a cause of theophylline toxicity. Official publication of the American College of Chest Physicians. 87 (6), 758-765.
Karim, A. Burns, T. Wearley, L. Streicher, J. Palmer, M. (1985). Food-induced changes in theophylline absorption from controlled-release formulations. Part I. Substantial increased and decreased absorption with Uniphyl tablets and Theo-Dur Sprinkle. Clin Pharmacol Ther. 38, 77-83.
Lopes, C M, Lobo J M, Pinto J F, Costa P C. Compressed matrix core tablet as a quick/slow dual-component delivery system containing ibuprofen. AAPS PharmSciTech. 2007 Sep 21;8(3):E76. doi: 10.1208/pt0803076. PMID: 17915826; PMCID: PMC2750572.
Maggi, L. Bruni, R. Conte, U. (2000). High molecular weight polyethylene oxides (PEOs) as an alternative to HPMC in controlled release dosage forms. International Journal of Pharmaceutics. 195, 229-238.
Meyer, RJ. Hussain, AS. (2005). Mitigating the Risks of Ethanol Induced Dose Dumping from Oral Sustained/Controlled Release Dosage Forms.Center for Drug Evaluation and Research. 1, 1-4.
Samuelov, Y. Dunbrow, M. Friedman, D. (1979). Sustained release of drugs from Ethylcellulose-polyethylene glycol films and kinetics of drug release. Journal of Pharmaceutical Sciences. 68, 325-329.
Schmidt, L. Dalhoff, K. (2002). Food-Drug Interactions. Drugs. 62 (10), 1481-1502.
Hypromellose vs Polyethylene Oxide in the Formulation Matrix “Mini” Tablet Systems
B Hussain and J Simon
Pharmacentral Laboratory
Faculty of Science and Technology, University of Central Lancashire, Preston, PR1 2HE