Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Sodium Lauryl Sulfate (also referred to as Sodium laurilsulfate or Sodium dodecyl sulfate) is a synthetic organic compound widely used as an excipient and in personal care products as an anionic surfactant. In the Ph.Eur, Sodium lauryl sulfate is described as a mixture of sodium alkyl sulfates and consists mainly of Sodium lauryl sulfate (chemical formula, CH3(CH2)10CH2OSO3Na). It is supplied as white or whitish-coloured crystals, powder or flaky material, with a slippery feel upon rubbing between fingers. It has a perceptible lipid odour.
Pharmacopoeial Compliance: USP-NF; Ph.Eur; B.P; J.P; I.P
Synonyms and Trade Names: Sodium Lauryl Sulphate; Sodium Lauryl Sulfate; SLS; Sodium Dodecyl Sulfate; SDS; Dodecyl Alcohol Hydrogen Sulfate, Sodium Salt Dodecyl Sulfate; Lauryl Sodium sulfate; Lauryl Sulfate, Sodium Salt; Monododecyl Sodium Sulfate; Sodium Dodecyl Sulfate; Sodium n-dodecyl Sulfate; Sodium Laurilsulfate; Sodium Monododecyl Sulfate; Sulfuric Acid Monododecyl Ester, Sodium Salt; Texapon KI2P; Elfan 240
Uses: Ionic Surfactant; Tablet Lubricant; Solubiliser; and Dissolution Rate Enhancer
Sodium Lauryl Sulfate also referred to as Sodium laurilsulfate or Sodium dodecyl sulfate is a synthetic organic compound widely used in personal care products as an anionic surfactant. It is similarly used in pharmaceutical products taking advantage of its surfactancy properties.
Sodium lauryl sulfate is prepared by reacting sulfuric acid with lauryl alcohol, followed with the addition of sodium carbonate to produce sodium lauryl sulfate. It is a negatively charged surfactant and one of the most detergents in personal and household products.
In the pharmacopoeia, Sodium lauryl sulfate is described as a mixture of sodium alkyl sulfates, consisting chiefly of sodium lauryl sulfate [CH3(CH2)10CH2OSO3Na]. The Ph.Eur and B.P specified the assay content of Sodium lauryl sulfate which should be not less than 85% of sodium alkyl sulfates calculated as C12H25NaO4S.
Sodium lauryl sulfate is supplied as a white or cream coloured solid substance that may sometimes appear pale yellow, especially after prolonged storage. It has a smooth, slippery (soapy) feel when rubbed between fingers and a perceptible odour of lipids.
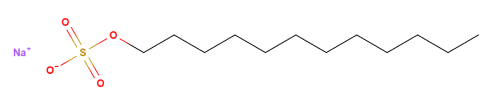
| Chemical name | Sulfuric acid monododecyl ester sodium salt |
| CAS Registry Number | [151-21-3] |
| Empirical Formula | C12H25NaO4S |
| Molecular Weight | 288.38 g/mol |
| EC Number | 205-788-1 |
| UNII Code (FDA) | 368GB5141J |
Sodium lauryl sulfate is an approved pharmaceutical excipient and is listed in all the major pharmacopoeia. It is also GRAS listed and included in the FDA Inactive Ingredients Database.
| Physical form | Solid, powder |
| Appearance | White or cream to pale yellow coloured crystals, flakes, or powder |
| pH value (1% w/w aqueous solution) | pH = 7.0-9.5 (1%w/v aqueous solution) |
| Critical micelle concentration | 8.2 mmol/l |
| Density | 1.07g/cm |
| HLB value | 40 |
| Interfacial tension | 11.8 mN/m (0.05% w/v solution) |
| Melting point | 204-207 oC |
| Moisture content | <5% |
| Solubility | Freely soluble in water |
| USP-NF | Ph.Eur | B.P | JP | |
| Authorised uses | Excipient | Excipient | Excipient | Excipient |
| Definition | specified | specified | specified | Specified |
| Identification | specified | specified | specified | specified |
| Characters | n/a | specified | specified | n/a |
| Alkalinity | specified | specified | specified | specified |
| Nonesterified alcohols | n/a | 4.0% | 4.0% | n/a |
| Sodium chloride and sodium sulfate combined content | ≤8.0% | ≤8.0% | ≤8.0% | ≤8.0% |
| Sodium chloride | n/a | n/a | specified | n/a |
| Sodium sulfate | n/a | n/a | specified | n/a |
| Heavy metals | ≤0.002% | n/a | n/a | ≤0.002% |
| Water | n/a | n/a | n/a | ≤5.0% |
| Unsulfated alcohols | ≤4.0% | n/a | n/a | ≤4.0% |
| Total alcohols | ≥59.0% | n/a | n/a | ≥59.0 |
| Assay (sodium alkyl sulfates) | n/a | ≥85.0% | ≥85.0% | n/a |
| Labelling | specified | n/a | n/a | n/a |
Key: n/a Specification is Not listed
*All claims in respect to the conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Sodium lauryl sulfate is widely used as an anionic surfactant, detergent, emulsifying agent, skin penetrant, tablet and capsule lubricant and wetting agent. It is used in solid dosage forms and topical products largely due to its surface-active properties. It may also be used to aid the dissolution rate of poorly soluble active substances during dissolution test measurements.
Examples of the different uses in various products are shown in the table below:
| Application | Recommended usage levels (%) |
| Anionic emulsifier in topical products | 0.5–2.5 |
| Surfactant, cleanser and foam generator in medicated shampoos | 2-10 |
| Skin cleanser in topical medical devices | 0.5-1 |
| Solubiliser in concentrations above the critical micelle concentration | >0.0025 |
| Alternative to Magnesium stearate as a tablet lubricant | 0.5–2.0 |
| Surfactant in film coating formulations | Up to 1% |
Sodium lauryl sulfate has been used in pharmaceutical and personal care products for several decades. While an approved excipient, Sodium lauryl sulfate is fairly harmful and acutely irritant to mucous membranes (eyes, upper respiratory tract and stomach). It has been shown that when skin is repeatedly exposed to Sodium lauryl sulfate, xeroderma (drying and cracking of skin) may occur. Inhalation of Sodium lauryl sulfate has the potential to damage respiratory tract tissue, and should therefore be avoided. Finally, Sodium lauryl sulfate is sensitising and may cause pulmonary allergies.
Toxicology: Human lethal oral dose is reported to be 0.5-5.0 g/kg body-weight. LD50 (rat, oral): 1.29 g/kg
Sodium lauryl sulfate is a fairly stable excipient under standard storage conditions. The shelf-life is reported as 24-36 months. In solution, Sodium lauryl sulfate may undergo hydrolysis to lauryl alcohol and sodium bisulfate when pH falls significantly (e.g < pH 2). Therefore, solutions should be used soon after preparation and/or not be exposed to extreme conditions.
Sodium lauryl sulfate can interact with cationic surfactants, leading to decreased activity. This can happen even when the concentrations of either species are very low. In formulated products, Sodium lauryl sulfate is incompatible with salts of polyvalent metal ions (e.g Aluminium, Zinc, and Magnesium).
Due to the inherent risks, the bulk material should be stored in a well-closed container away from strong oxidizing agents in a cool, dry place. When handling the material, workers should observe standard SHEQ protocols in line with the circumstances and quantity of material being processed. Inhalation and contact with the skin and eyes should be avoided. Use of eye protection, gloves, and other suitable PPE are advised. The workspace should provide adequate ventilation and/or a dust respirator should be used. Consult the MSDS for more detailed information.
A sustainability score has not been provided at this point.
Stepan Corporation
[2] J. Hanaee, Y. Javadzadeh, S. Taftachi, D. Farid, A. Nokhodchi, The role of various surfactants on the release of salbutamol from suppositories, Farmaco, 59 (2004) 903-906.
[3] G. Heinicke, J.B. Schwartz, The influence of surfactants and additives on drug release from a cationic Eudragit coated multiparticulate diltiazem formulation, Pharm Dev Technol, 12 (2007) 381-389.
[5] P.J. Sheskey, W.G. Cook, C.G. Cable, A. American Pharmacists, Handbook of pharmaceutical excipients, 2017.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.