Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Isomalt is a sugar alcohol (polyol) composed of a mixture of 6-O-α-D-glucopyranosyl-D-sorbitol (1,6-GPS) and 1-O-α-D-glucopyranosyl-D-mannitol (1,1-GPM) that are simultaneously obtained by hydrogenation of isomaltulose. It is supplied as a white or almost white powder or granular or crystalline substance with a pleasant sugar-like taste (approximately 50 – 60% of that of sucrose).
Synonyms & Trade Names: Isomalt; Hydrogenated Isomaltulose; E953; IsoMaltidex; galenIQ; Palatinit
Regulatory Compliance: USP-NF; Ph.Eur; J.P; B.P; I.P; FCC
Uses & Applications: Wet Granulation Binder; Suspending Agent or Viscosity-increasing Agent, and Solubiliser
Isomalt is a polyol (sugar alcohol) made up of a mixture of 1,6-GPS and 1,1-GPM. 1,6-GPS crystallises without water and is more soluble than 1,1-GPM. By altering the ratio of the two forms, the solubility and crystal water content can be adjusted. For instance, galenIQ 720 isomalt has a GPM:GPS ratio of 1:1 while galenIQ 721 has a GPM:GPS ratio of 1:3.
Isomalt was discovered in the 1950s and has grown to become the number one sugar substitute. It is known to occur naturally in honey and sugar cane, however, commercial sources are obtained from Sucrose (mainly sugar beet) in a process that converts the sucrose into isomaltulose (palatinose) in an enzyme-catalysed reaction. The isomaltulose is then catalytically hydrogenated to Isomalt. The enzyme rearranges the bonds between Glucose and Fructose (in sucrose) while the hydrogenation step introduces two hydrogens in the fructose portion of the disaccharide. These changes make Isomalt very stable, both chemically and enzymatically, than sucrose, which is the reason for its many health benefits (low or no impact on blood glucose levels, non-cariogenicity, and low calories).
Isomalt is supplied as a white or almost white powder or granular or crystalline substance. It has no odour and is non-hygroscopic. It tastes very much like sugar and has a mild sweetness approximately 50 – 60% of that of sucrose.
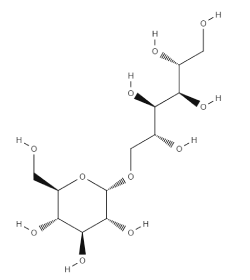
| Chemical Name and CAS Registry Number | Isomalt [64519-82-0]
1,6-GPS (6-O-α-D-glucopyranosyl-D-sorbitol [534-73-6] 1,1-GPM (1-O-α-D-glucopyranosyl-D-mannitol dihydrate [20942-99-8] |
| Empirical Formula and Molecular Weight | C12H24O11 – 344.32 (anhydrous)
C12H24O11.2H2O – 380.32 (for dihydrate) |
| EC Number | 613-617-0 |
| UNII Code (FDA) | S870P55O2W |
Isomalt is an approved pharmaceutical excipient and is currently listed in all the major pharmacopoeia. It is also GRAS listed and accepted as a food additive in Europe. Isomalt is currently approved in more than 80 countries worldwide, including the United States, the European Union, the United Kingdom, Japan, Australia, New Zealand and a number of countries in Asia and South America. The top producers in the U.S. are Beneo and Cargill Inc.
| Physical form | Solid, powder |
| Appearance | White or almost white powder, granular or crystalline material |
| Angle of repose | 30 – 40o |
| Moisture content | ≤ 5% under ambient conditions |
| Compressibility | Compression characteristics vary by grade of Isomalt |
| Poured density | 0.40 – 0.90 g/cm3 |
| Tapped density | 0.40 – 0.80 g/cm3 |
| True density | 1.52g/cm3 for 1, 6 –GPS
1.47g/cm3 for 1,1-GPM |
| Flow ability | Powder may be cohesive while granules are free-flowing |
| Glass transition temperature | 63 oC (1:3 mixture of 1,1-GPM and l,6-GPS)
68 oC (1,1-GPM) 59 oC (1,6-GPS) |
| Hygroscopicity | Isomalt is not hygroscopic under ambient conditions however above 85% RH (25 oC) it absorbs moisture |
| Melting point | Dependent on grade:
141 – 161 oC (1:3 blend of 1,1-GPM and 1,6-GPS 166 – 168 oC (1,6-GPS) 168 – 171 oC (l,1-GPM) |
| Particle size distribution | 90% >100µm for galenIQ 720
58% >20 µm for galenIQ 800 99% >200µm for galenIQ 960 |
| pH | 3 – 10 |
| Solubility | 20 – 40% at 25oC |
| USP-NF | Ph.Eur | |
| Name | Isomalt | Isomalt |
| Authorised use | Excipient | Excipient |
| Definition | specified | specified |
| Identification | A
B |
A
B |
| Appearance | A white or almost powder or granules, freely soluble in water | A white or almost powder or granules, freely soluble in water |
| Conductivity | ≤20 µS.cm-1 | ≤20 µS.cm-1 |
| Water | ≤7.0 % | ≤7.0 % |
| Heavy metals | n/a | ≤10 µg/g |
| Reducing sugars | ≤0.3% | ≤0.3% |
| Nickel | ≤1 ppm | ≤1 ppm |
| Lead | n/a | ≤0.5 ppm |
| Related compounds | Specified | specified |
| Assay | 98.0-0-102.0% | 98.0-102-0% |
| Labeling | specified | n/a |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Isomalt is used as a coating agent; granulation aid; medicated confectionery base; sweetening agent, and tablet and capsule diluent. Being a non-cariogenic excipient it has been used in a variety of pharmaceutical preparations such as tablets or capsules, coatings, sachets, suspensions, and effervescent tablets. In order to satisfy these requirements, manufacturers avail the material in several grades which vary by particle size and bulk density. For example, Beneo supplies the following grades:
It is also used widely in lozenges, sugar-free chewing gum, hard-boiled candies, and as a sweetening agent in confectionery. It dissolves more slowly in the mouth compared to other polyols, meaning that products made with Isomalt tend to have a longer-lasting sweet taste in the mouth. Finally, Isomalt tends to absorb very little water, therefore products formulated with it are not sticky. This improves packaging, and ultimately, shelf-life.
Perhaps most important property of Isomalt is its excellent and smooth taste, which is very sugar-like but without its ill effects. Products formulated with Isomalt have been reported to have the same texture and appearance as those made with sugar making Isomalt an excellent candidate for sugar-replacement (calorie-reduced) pharmaceutical products. To round off the sweetness, it can be used in combination with intense sweeteners, such as Sucralose, and Neotame: the isomalt provides the bulk, texture and mild sweetness while the intense sweetener brings the sweetness up to the level that would be achieved with sugar alone.
Note that compression of Isomalt without lubrication is problematic, and issues such as die wall sticking, capping, and lamination are often experienced. Therefore, the addition of a lubricant (such as Magnesium stearate) is recommended to prevent these problems.
Isomalt has been used for several years in various products, including hard candies, toffees, chewing gums, chocolates, baked goods, dietary supplements, cough drops, and throat lozenges. It is found to occur naturally in honey and sugar cane. The US FDA considers it to be a generally safe and non-toxic material. Studies examining Isomalt’s toxicological and metabolic effects have been summarized in a WHO report prepared by the FAO/WHO Expert Committee (JECFA), leading to the conclusion of acceptable daily intake of not specified.
Due to the high stability of the glycosidic bonds between the mannitol or sorbitol moiety and the glucose residues, acid-catalysed hydrolysis and absorption of Isomalt in the small intestine are minimal. Therefore, there is no marked increase in the blood glucose levels following oral ingestion, and glycemic response is low. For this reason, Isomalt is suitable for diabetic patients. The majority of isomalt is fermented by gut flora into free fatty acids.
Isomalt is not cariogenic since it is not fermented by bacteria present in the mouth; therefore there it carries minimal risk of organic acid production capable of attacking dental enamel. However, like most polyols, it may cause gastric distress when consumed in very large quantities (above 20-30 g per day). It may cause upset stomachs because of the tendency to cause osmotically-induced diarrhoea.
Isomalt is reported to exhibit very good thermal and chemical stability. No changes in the molecular structure are observed when melted, and it is resistant to acids and microbial attack. Isomalt is also non-hygroscopic, and at ambient conditions (25 °C) it absorbs very little moisture. Isomalt does not undergo browning reactions; it has no reducing groups, and therefore it does not react with other formulation ingredients (e.g. with amines in Maillard reaction). Thus, if the material is under standard ambient conditions, Isomalt can be expected to remain chemically stable for several years. Manufacturers, however, recommend that Isomalt be stored in an unopened container at 20 °C and <60% RH. The material should be re-evaluated after 3 years.
When handling Isomalt, workers should observe established SHEQ protocols appropriate to their individual circumstances and the quantity of material being processed. The wearing of eye protection, gloves, and a dust mask or respirator are recommended for effective protection from the irritation of the airway and/or skin.
A sustainability score has not yet been computed by the Excipients Forum for Isomalt.
Cargill Corporation
Beneo-Palatinit GmbH
[5] Z. Sáska, J. Dredán, E. Balogh, O. Luhn, G. Shafir, I. Antal, Effect of isomalt as a novel binding agent on compressibility of poorly compactable paracetamol evaluated by factorial design, Powder Technology, 201 (2010) 123-129.
[6] A.-K. Tuderman, C.J. Strachan, A.M. Juppo, Isomalt and its diastereomer mixtures as stabilizing excipients with freeze-dried lactate dehydrogenase, International Journal of Pharmaceutics, 538 (2018) 287-295.
Isomalt is a polyol, which is a type of sugar alcohol. Polyols are saccharides in which the aldehyde group is replaced by a hydroxyl group. Polyols are ubiquitous in nature, and include compounds such as glucitol, xylitol, and maltitol, which are used to provide sweet taste in food products.
Isomalt occurs naturally in honey and sugar cane. The commercial material is however produced from sucrose via a process that combines chemical synthesis and enzymes, thus it is technically a nature-derived material. It does not have colouring agents, flavours, preservatives, antioxidants, intense sweeteners or any other materials added in order to fulfil classification as a direct food additive.
Isomalt is regulated as food additive in most countries worldwide. It is Generally Recognized As Safe by self-determination (United States). It is manufactured in compliance with the applicable mandatory criteria such as FAO/WHO (Codex Alimentarius), the Food Chemicals Codex and Regulation (EC) No 231/2012 (European Union).
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.