Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Glyceryl Monostearate (GMS) is a mixture of monoacylgcerols, mostly monosteroylglycerol, together with quantities of di-and triacylglycerols. In the Ph.Eur, Glyceryl monostearate is distinguished into different grades, namely Type I, II and III depending on their fatty acid composition. When supplied as an excipient, Glyceryl monostearate occurs as a hard, waxy mass or greasy powder, flakes or beads.
Synonyms and Trade Names: Glyceryl Monostearate; Glycerol Monostearate; Glyceryl Monostearate 40-55; 2,3-Dihydroxypropyl Octadecanoate; Glycerine monostearate; Glycerol monostearate; Monoglyceride; Glycerol stearate; GMS; Capmul GMS; HallStar GMS; Imwitor 900; Protachem CGM-450; Stepan GMS
Pharmacopoeial Compliance: USP-NF; Ph.Eur; J.P; B.P, IP
Uses and Applications: Emollient; Emulsifying Agent; Solubilizing Agent; Stabilizing Agent, and Tablet and Capsule Lubricant
Glyceryl Monostearate (commonly abbreviated as GMS) is a lipoid material made up of a mixture of various proportions of monoacylgcerols, mostly monosteroylglycerol, together with quantities of di-and triacylglycerol. It exists in the form of a white or cream, hard, waxy mass or greasy powder, flakes or beads. Although the names glyceryl monostearate and mono-and di-glycerides are used for several esters of long-chain fatty acids, Glyceryl Monostearate esters can be grouped into the following two main categories:
Details of the type and content of fatty acids incorporated in Glyceryl monostearate 40-55 Type I, II and III are shown below:
| Glyceryl monostearate | Fatty acid used in manufacturing | Composition of fatty acids | |
| Stearic acid | Sum of palmitic and stearic acids | ||
| Type I | Stearic acid 50 | 40.0 – 60.0% | ≤90.0% |
| Type II | Stearic acid 70 | 60.0 – 80.0% | ≤90.0% |
| Type III | Stearic acid 95 | 80.0 – 99.0% | ≤96.0% |
Glyceryl monostearate is manufactured by reacting Glycerin with triglycerides obtained from animal or vegetable sources, producing a mixture of monoglycerides and diglycerides. The diglycerides are subsequently reacted to produce the 90% monoglyceride grade. A different production pathway involves the combination of Glycerol with stearoyl chloride.
Finally, Glyceryl monostearate is a somewhat peculiar raw material because its composition, and therefore the physical properties, do vary remarkably from manufacturer to manufacturer. This is mainly because the starting materials used in the synthesis of Glyceryl monostearate are not pure substances, thus the products obtained from the processes contain a mixture of esters, such as palmitate and oleate.
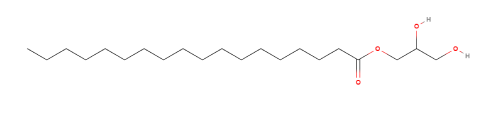
| Chemical Name | Octadecanoic acid, monoester with 1,2,3-propanetriol |
| CAS Registry Number | [31566-31-1] |
| Empirical Formula and | C21H42O4 |
| Molecular Weight | 358.6 |
| EC Number | 250-705-4 |
| UNII COde | 230OU9XXE4 |
Glyceryl monostearate is an approved pharmaceutical excipient. It is listed in all major pharmacopoeia. The USP-NF also includes a specification for mono and di-glycerides, corresponding to Glyceryl monostearate 40-55 in the Ph.Eur. It is currently listed in the US FDA Inactive Ingredients Database (oral, ophthalmic, otic, rectal, topical, transdermal, and vaginal formulations) and is GRAS listed. Glyceryl monostearate is also an approved food additive and cosmetic ingredient, and the Food Chemicals Codex (FCC) also contains a specification for Glyceryl monostearate.
Glyceryl monostearate is available in many different grades whose physical properties vary by the commercial producer. A number of grades are self-emulsifying grades due to the presence of small concentrations of Sodium lauryl sulfate and other surfactants. Many grades are purpose-made to particular applications or customer specifications. The following is only a generalised summary:
| Physical form | Solid, powder |
| Appearance | White or whitish, waxy solid |
| HLB Value | 3 (IMWITOR® 900 K) |
| Flash point | 240oC |
| Melting point | 55-60 oC |
| Boiling point |
238-240 oC |
| Relative density | 1.03 g/ml |
| Solubility | Insoluble in water |
Glyceryl monostearate 40-55/Mono- and Di-Glycerides
| USP-NF | Ph.Eur | J.P | |
| Official name | Mono- and Di-glycerides
|
Glyceryl Monostearate 40-55 | Glyceryl Monostearate |
| Authorised use | Excipient | Excipient | Excipient |
| Definition | specified | specified | specified |
| Identification | n/a | specified | specified |
| Characters | n/a | specified | n/a |
| Acid value | ≤4.0 | ≤3.0 | ≤15.0 |
| Iodine value | 90.0-110.0%(b) | ≤3.0 | ≤3.0 |
| Hydroxyl value | 90.0 -110.0%(b) | n/a | n/a |
| Saponification value | 90.0-110.0%(b) | 158-177 | 157-170 |
| Melting point | ≥55oC | n/a | ≥55oC |
| Residual on ignition | ≤0.1% | ≤0.1% | ≤0.1% |
| Acidity or alkalinity | n/a | n/a | + |
| Free glycerin | ≤7.0% | ≤6.0% | – |
| Composition of fatty acids | n/a | specified | n/a |
| Heavy metals | ≤0.001% | n/a | n/a |
| Arsenic | ≤3ppm | n/a | n/a |
| Nickel | n/a | ≤1ppm | n/a |
| Water | n/a | ≤1.0% | n/a |
| Assay (monoglycerides) | specified | n/a | n/a |
| Labelling | specified | n/a | n/a |
Glyceryl monostearate 90%
| USP-NF | J.P | |
| Official name | Glyceryl Monostearate | Glyceryl Monostearate |
| Authorised use | Excipient | Excipient |
| Definition | specified | specified |
| Identification | n/a | specified |
| Acid value | ≤6.0 | ≤15.0 |
| Iodine value | ≤3.0 | ≤3.0 |
| Hydroxyl value | 290-330 | specified |
| Saponification value | 150-165 | 157-170 |
| Melting point | ≥550C | ≥550C |
| Residue on ignition | ≤0.5% | ≤0.1% |
| Acidity or alkalinity | n/a | specified |
| Limit of free glycerin | ≤1.2% | specified |
| Heavy metals | ≤0.001% | specified |
| Assay (monoglycerides) | ≥90.0% | specified |
| Labelling | specified | n/a |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Glyceryl monostearate is principally used as an emollient, mild emulsifying agent, solubilizing agent, stabilizing agent, and tablet and capsule lubricant. Owing to its lipid nature as well as the availability of many different grades, Glyceryl monostearate exhibits thickening, emulsifying and protective properties, making it a versatile excipient across multiple applications and dosage forms.
A summary of the different applications of Glyceryl monostearate in food, pharmaceutical, and cosmetic applications is outlined below:
Incidences of polymorph formation when Glyceryl monostearate is used in product formulation are an important consideration. Generally, the α-form tends to disperse easily and foams, rendering it useful as an emulsifying agent. The β-form, being more stable, is suitable for use in sustained-release matrices.
Note that Glyceryl monostearate is not an efficient emulsifier. Its usefulness resides in the fact that it is a useful emollient and co-emulsifier. It is readily emulsified by common emulsifying agents and by the incorporation of other fatty materials into the formulation. When added to creams, Glyceryl monostearate imparts to creams smoothness, and fineness, while improving the formulation stability.
About Glyceryl Monostearate – Self-emulsifying
Self-emulsifying Glyceryl monostearate is a grade of Glyceryl monostearate to which an emulsifying agent has been added. A specification for self-emulsifying Glyceryl monostearate was previously included in the Ph.Eur (it still remains in the B.P). This material is mainly used as an emulsifying agent for oils, fats, solvents, and waxes.
Glyceryl monostearate is commonly used in pharmaceutical products, cosmetic formulations, and baked foods. It is generally regarded as a nontoxic and nonirritant material. On the basis of the available animal data and clinical experience, health authorities have determined that Glyceryl monostearate is safe for oral and topical use in humans in the present practices of use and concentration.
Toxicology: LD50 (mouse, IP): 0.2g/kg
Glyceryl monostearate is a relatively stable and non-reactive excipient. Shelf life is assigned as 24-36 months. However, if stored at warm temperatures, Glyceryl monostearate is reported to increase in acid value due to the saponification (the esters react with trace amounts of water). The addition of an effective antioxidant, for instance, Butylated hydroxytoluene and Propyl gallate, can mitigate this phenomenon. Nevertheless, Glyceryl monostearate should be stored in a tightly-closed container and placed in a cool, dry place, away from direct heat and light.
When handling the bulk material, workers should observe established SHEQ protocols applicable in their institutions depending on their unique circumstances and the quantity of material being processed.
A sustainability score for Glyceryl monostearate has not been computed. However, Glyceryl monostearate uses trigylcerides derived from palm oil, which is a major driver of deforestation of some of the world’s most biodiverse forests. Even Glyceryl monostearate claimed to be COSMOS or RSPO certified includes material obtained from the same destructive practices. For this reason, some campaigners do not believe COSMOS and RSPO principles are effective.
IOI Oleo
Mosselman Oleo
Gattefossé
[3] R. O’laughlin, C. Sachs, H. Brittain, E. Cohen, Effects of variations in physicochemical properties of glyceryl monostearate on the stability of an oil-in water cream, J Soc Cosmet Chem, 40 (1989) 215-229.
[4] M.A. Darji, R.M. Lalge, S.P. Marathe, T.D. Mulay, T. Fatima, A. Alshammari, H.K. Lee, M.A. Repka, S.N. Murthy, Excipient stability in oral solid dosage forms: A review, AAPS PharmSciTech, 19 (2018) 12-26.
[5] G.K. Bolhuis, N. Anthony Armstrong, Excipients for direct compaction—an update, Pharmaceutical Development and Technology, 11 (2006) 111-124.
[7] T.U.S.P. Convention, Food Chemicals Codex (11th Edition), (2018).
[8] P.J.C. Sheskey, Walter G; Cable, Colin G, Handbook of Pharmaceutical Excipients – 8th Edition, Pharmaceutical Development and Technology, 18 (2017) 544-544.
[9] V. Jannin, Y. Cuppok, Hot-melt coating with lipid excipients, International Journal of Pharmaceutics, 457 (2013) 480-487.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.