Suspending agents are excipients added to disperse systems in order to maintain particulate ingredients in suspension or to prevent other forms of physical instability. In this technical note, we provide an introductory review of what suspending agents are, and outline the function they play in pharmaceutical formulations.
Definition of a Pharmaceutical Suspending Agent
Pharmaceutical suspending agents are a class of excipients added to disperse systems to ensure that any solid particles in the formulation are kept uniformly distributed within the continuous phase, thereby maintaining physical stability of the product.
Pharmaceutical dispersions include suspensions, creams and aerosols. They typically comprise dispersions of insoluble solids (drug and/or excipients) in an aqueous or non-aqueous liquid medium, known as the continuous phase.
Depending on the particle size of the disperse phase, disperse systems can be further categorised as:
- Colloidal (the dispersed particles are in the submicron range), and
- Coarse (dispersed particles are above colloidal size).
Note that the term ‘suspending agent’ is frequently used interchangeably with another, closely related term, ‘thickening agent’.
As we will see, while having some commonalities the two excipients groups are different. Very simply, thickeneing agents can be used as suspending agents but not all suspending agents are thickening agents.
This is best illustrated by the following commonly used OTC products:
Pepto Bismol® is an oral suspension of bismuth salicylate (active). It is formulated with aluminium magnesium silicate, methylcellulose, and gellan gum, which are the materials used to hold the active ingredient in suspension by increasing viscosity (thickeners).
Gaviscon® is an oral suspension of sodium bicarbonate, calcium carbonate, and sodium alginate. The sodium alginate functions both as a suspending excipient and active ingredient.
Calpol® is an oral suspension of paracetamol formulated with maltitol and sorbitol (syrups), microcrystalline cellulose, sodium carboxymethylcellulose, and xanthan gum, as the suspending agents. Suspension of the active is achieved through thickening of the dispersing phased.
In order to appreciate the utility of suspending agents, it’s necessary to quickly review the technical aspects of pharmaceutical disperse systems.
The process of dispersing solid particles in a liquid creates several problems, including:
- Creaming
- Sedimentation
- Flocculation
- Caking
- Particle growth, and
- Adhesion onto the walls of the container (in some formulations)
The above phenomenon constitute physical instabilities, and are generally dependent on three main factors:
- Differences in density between the dispersed particles and the dispersion medium (∆ρ)
- Particle size (radius) of the dispersed phase r
- Viscosity of the dispersion medium (η)
The interrelationship between these three factors is given by Stokes’ law, which is shown below:
v=2gΔρr²/9η
where v is the rate of particle settling (or creaming, for that matter).
According to Stokes law, instability can be reduced by reducing particle size, decreasing density differences between the two phases or increasing the viscosity of the continuous phase.
It is worth knowing that particles will still be able to move about in the dispersion, however, the rate and intensity of collisions will be minimised. Furthermore, disperse systems are highly dynamic – dispersed particles frequently contact each other as a result of the following phenomenon:
- Brownian motion
- Creaming
- Sedimentation due to gravitational forces
- Convection
Given enough time, and in the absence of any other mitigating circumstances, the dispersed particles eventually come into close contact and may coalesce, form bonds and cake, destroying the balance of the disperse system.
Whether dispersed particles reach this point of no return depends on two factors:
- Forces of attraction or repulsion, and
- Nature of the surface of the dispersed particle
This is where suspending agents come into play, i.e they help minimise dispersed particle flux (sedimentation, creaming or convection) or interfere with particle-particle attraction.
We can therefore define pharmaceutical suspending agents as a specific category of excipients added to disperse systems to minimise disperse phase coalescence and instability.
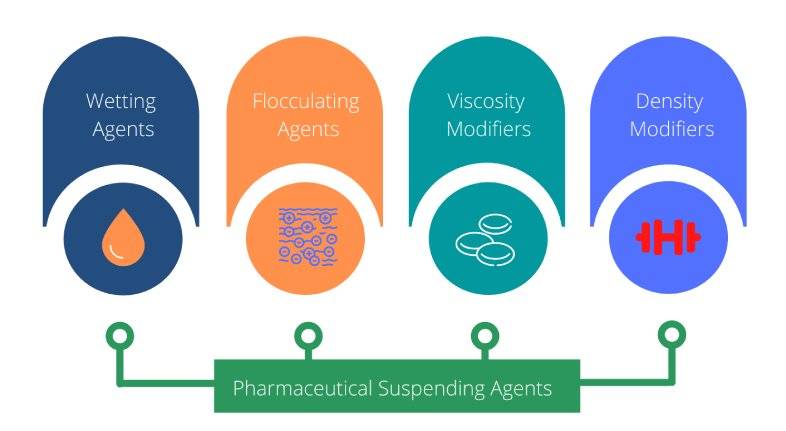
Pharmaceutical suspending agents can be grouped into four main categories as outlined below:
- Wetting agents
- Flocculating agents
- Viscosity modifiers (thickeners)
- Density modifiers
Types of Pharmaceutical Suspending Agents
A). Wetting agents
For the disperse phase to freely and distribute in the continuous phase, it needs to be wetted by the liquid. The presence of entrapped air pockets on the particle surface or if the particles are hydrophobic is a hindrance to dispersion.
Wettability can be enhanced through the reduction of the interfacial tensions, namely solid-liquid and liquid-vapour interfaces.
1. Surface active agents
Surface active agents (surfactants for short) are excipients that lower the surface tension (or interfacial tension) between two liquids or between a liquid and a solid.
Surfactants with HLB values between 7 and 9 have been shown to be suitable wetting agents in pharmaceutical disperse systems.
This is because the apolar groups of the surfactant are able to adsorb onto hydrophobic parts of formulation particle while the polar groups project into the aqueous medium, resulting in a lowering of the interfacial tension between the solid and the liquid.
2. Hydrophilic colloids
Hydrophilic colloids are high molecular polysaccharide polymers or clays. They include acacia, bentonite, tragacanth, alginates and cellulose derivatives.
Being hydrophilic, they are able to coat particles to impart hydrophilic character to the disperse phase. In addition, hydrophilic colloids may also increase viscosity of the continuous phase, reducing the rate of sedimentation of particles.
3. Solvents
Common polar pharmaceutical solvents such as glycerol, propylene glycol and polyethylene glycol and alcohol are highly water-miscible and are able to reduce the liquid-air interfacial tension.
They enable water to infiltrate deep into power agglomerates, displacing entrapped air and enabling wetting of the dispersion to take place.
B). Flocculants
A well-formulated disperse system is one that exhibits the correct degree of flocculation. Systems that are underflocculated generally tend to settle and sediment very rapidly although the compacts formed are loose and redispersion is possible.
On the other hand, overflocculation results in products that while settle slowly the sediments pack tightly such that redispersion is not possible.
By controlling the degree of flocculation, it’s possible to reach a happy medium – in which there is a degree of flocculation and also deflocculation.
This can be achieved through, not only through particle size control and viscosity-modification, but also by using flocculating agents, including electrolytes, ionic surfactants and polymer flocculating agents.
1. Electrolytes
The addition of inorganic electrolytes to the solution changes the zeta potential of the dispersed particles, and provided this is lowered sufficiently enough, will produce a flocculated system.
2. Surfactants
Ionic surfactants can also be used to create flocculation in a disperse system by neutralising particle charges and creating a deflocculated system.
3. Polymeric flocculants
Several polymeric excipients are capable of forming gel-like networks in disperse systems thereby adsorbing onto and trapping dispersed particles and holding them in a flocculated state.
These materials are mainly hydrocolloids although not always, and include:
C). Viscosity modifiers
The deal disperse system is one that displays pseudoplastic or thixotropy behaviour, that is, exhibit high apparent viscosity at low shear rates.
Upon storage, the dispersed particles either settle slowly or, and preferably, remain suspended. When the product is sheared, for instance, when shaken by the consumer, the high apparent viscosity of the formulation should fall sufficiently for product to be dispensed easily.
The various materials currently used as viscosity modifiers are outlined below:
1. Polysaccharides
Examples of polysaccharide-based viscosity modifying excipients include the following:
2. Water-soluble cellulose ethers
Several cellulose ethers have the ability to increase viscosity of aqueous systems in which they are dispersed. They include:
- Methylcellulose
- Hydroxyethylcellulose
- Sodium carboxymethylcellulose, and
- Microcrystalline cellulose
- Hypromellose
The viscosity-increasing properties of cellulose ethers depends on the molecular weight and degree of substitution.
3. Hydrated silicates
Hydrated silicates are naturally-occurring siliceous clays that exist as colloids in water. This group of pharmaceutical grade clays includes:
- Bentonite
- Hectorite
- Magnesium aluminium silicate (VEEGUM®)
Hydrated silicate materials exhibit thixotropic behaviour at low concentration in water and as gels at high concentration.
4. Carbomers
Carbomers are high molecular weight cross-linked polyacrylic acid polymers that swell in water to form viscous hydrogels depending on the degree of cross-linking.
Among carbomer excipients, carbomer 940 is the most commonly used suspending excipient in both topical and oral products.
If you would like to learn more about carbomers, here is a link to an article that gives an overview on these important excipients:
D). Density modifiers
From probing Stokes’ law above, it is clear that if the densities dispersed and dispersing medium are of the same magnitude sedimentation would be significantly slowed down.
Thus, changing the density of the dispersing medium, for example, addition of glycerol, propylene glycol, polyethylene glycol or sucrose-based syrups, can significantly modify densities and leveraged to control instability.
You read more about viscosity modifying excipients through this link:
Summary of Pharmaceutical Suspending Agents
Many pharmaceutical products are formulated as disperse systems in which particulate solids (active ingredients and/or excipients) are distributed throughout a continuous or disperse phase. Owing to the tendency towards settling or creaming by the disperse phase, the use of suspending agents is mandated.
There are many different types of pharmaceutical suspending agents; they can be grouped into four main classes depending on the mechanism of function:
- Wetting agents
- Flocculating/deflocculating agents
- Viscosity modifiers
- Density modifiers
Careful selection of suspending agents is essential in order to have a product that ensures the disperse phase does not settle rapidly, the particle do not settle into a cake, the system is easily re-dispersed into a uniform mixture when shaken, is easy to dispense from the container or use by the consumer.
Sources and Further Reading
- Schuch A., Schuchmann H., Gaukel V. (2015) Rheology of Emulsions. In: Drioli E., Giorno L. (eds) Encyclopedia of Membranes. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-40872-4_1884-1.
- Moreton R.C. (2010) Commonly Used Excipients in Pharmaceutical Suspensions. In: Kulshreshtha A., Singh O., Wall G. (eds) Pharmaceutical Suspensions. Springer, New York. https://doi.org/10.1007/978-1-4419-1087-5_3.