Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Carboxymethylcellulose is the sodium salt of a carboxymethyl ether of cellulose obtained from plant material. In essence, it is a chemically modified cellulose that has a carboxymethyl ether group (-O-CH2-COO-) bound to some of the hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone. It is available in different degrees of substitution, generally in the range 0.6 – 0.95 derivatives per monomer unit, and molecular weights. Commercial grades of Carboxymethylcellulose sodium are supplied as white to almost white, odourless, tasteless, granular powders.
Synonyms and Trade Names: Carboxymethylcellulose sodium; Carmellose Sodium; Carboxymethylcellulose Sodium; Sodium Carboxymethylcellulose; SCMC; CMC-Na; Aquasorb; Blanose; Carbose D; Cellulose Gum; CMC sodium; E466; Finnfix; SCMC; Tylose CB; Tylose MGA; Walocel C
Pharmacopoeial Compliance: Ph. Eur; USP-NF; BP; IP
Uses and Applications: Suspending Agent; Tablet and Capsule Disintegrating Agent; Tablet Binder; Viscosity-increasing Agent; and Film Former (Coating Agent)
Carboxymethylcellulose (technically, Carboxymethylcelluloses) is a family of chemically modified cellulose derivatives containing the carboxymethyl ether group (-O-CH2-COO-) bound to some of the hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone. When Carboxymethylcellulose is recovered and presented as the Sodium salt, the resulting polymer is what is known as Carboxymethylcellulose sodium, and has the general chemical formula, [C6H7O2(OH)x(OCH2COONa)y]n.
Carboxymethylcellulose was discovered shortly after Word War 1 and has been produced commercially since the early 1930s. It is produced by treating cellulose with an aqueous sodium hydroxide solution followed by monochloroacetic acid or its sodium salt. In a parallel reaction two by-products, sodium chloride and sodium glycolate, are produced. Once these by-products are removed, high purity Sodium carboxymethylcellulose is obtained. As a general rule, the obtained material has a slight excess of sodium hydroxide and has to be neutralised. The neutralisation endpoint can affect the properties of the material. In the final step, the material is dried, milled to the desired particle size, and packaged.
Food and pharmaceutical grade Carboxymethylcellulose is required by law to contain not less than 99.5% pure CMC and a maximum of 0.5% of residual salts (sodium chloride and sodium glycolate). The degree of substitution (DS) can vary between 0.2-1.5, although it is generally in the range of 0.6-0.95. The DS determines the behaviour of CMC in water: Grades with DS >0.6 form colloidal solutions in water that are transparent and clear, i.e the higher the content of carboxymethyl groups, the higher the solubility and smoother the solutions obtained. CMC with a DS below 0.6 tends to be only partially soluble.
Carboxymethylcellulose sodium is available as a white to almost white, odourless, tasteless, granular powder.
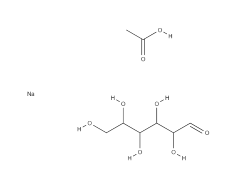
| Chemical Name | Cellulose, carboxymethyl ether, sodium salt |
| CAS Registry Number | [9004-32-4] |
| Empirical Formula | [C6H7O2(OH)x(OCH2COONa)y]n
x = 1.5-2.0, y = 0.2-1.0. y = degree of substitution |
| Molecular Weight | 250 000 (average) |
| Regulatory Status | PhEur; USP-NF; JPE; FCC |
Carboxymethylcellulose sodium is an approved pharmaceutical excipient. It is currently listed in all the major pharmacopoeia, including the USP-NF; Ph.Eur; J.P, I.P and ChP. It is also GRAS listed and included in the FDA Inactive Ingredients Database. Carboxymethylcellulose sodium is approved as a food additive in Europe (E466).
| Physical Form | Solid, powder
Particle size >75µm (40-75%); >500µ (≤2%) |
| Appearance | White or whitish/cream fibrous powder |
| Odour | Odourless |
| pH (1% solution) | 6.5-8.0 |
| Equilibrium moisture content (ambient) | 8% |
| Density (poured) | 0.50-0.55 g/ml depending on source and grade |
| Density (tamped) | 0.76-0.79 g/ml depending on source and grade |
| Melting point | N/A
Decomposes at 252 oC. |
| Solubility | Easily hydrated in water at all temperatures to produce viscous clear solutions. Solubility (rate) influenced by molecular weight and degree of substitution |
| Viscosity | Depending on grade, viscosity of Carboxymethylcellulose sodium varies between 200 to >11000 cP (1-4% concentration). Increasing the gum’s concentration produced an increase in viscosity |
| Test Specification | Specification | Reference |
| Definition | Specified | USP-NF/Ph.Eur |
| Characters | White or almost white, granular powder | USP-NF/Ph.Eur |
| Identification | Specified | USP-NF/Ph.Eur |
| Appearance of solution | Specified | USP-NF/Ph.Eur |
| pH | 6.0- 8.0 | USP-NF/ Ph.Eur |
| Viscosity | Specified | USP-NF/Ph.Eur |
| Sodium glycollate | Specified | USP-NF/Ph.Eur |
| Chlorides | 0.25% | USP-NF/Ph.Eur |
| Heavy Metals | ≤20ppm | USP-NF/Ph.Eur |
| Loss on Drying | ≤10.0 | USP-NF/Ph.Eur |
| Sulphated Ash | ≤20 – 33% | USP-NF/Ph.Eur |
| Labelling | Specified | USP-NF/Ph.Eur |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Carboxymethylcellulose sodium is one of the most versatile of all water-soluble colloids. It serves as a film former in polymer coatings; stabilizing agent; suspending agent; tablet and capsule disintegrant; tablet binder; viscosity-increasing agent; and moisture binder. However, the main applications areas of Carboxymethylcellulose sodium are in oral and topical formulations primarily due to its viscosity-increasing properties. It is used to form viscous solutions which are then utilised to maintain powders in suspension, which is relevant to oral products. It has also been occasionally used as a tablet binder and disintegrant, and to stabilize emulsions.
A summary of the different uses of Carboxymethylcellulose sodium and their typical levels are summarised below:
| Function | Recommended level (% w/w) |
| Thickening agent (oral and topical products) | 0.1-1.0 |
| Gelling agent (topical products) | 2.0-6.0 |
| Suspending agent in oral liquids | 0.1-1.0 |
| Wet granulation binder | 3.0-4.0 |
Medium-viscosity grades of Carboxymethylcellulose sodium (at median concentration of 4.5%) are used to form gels. In topical medical devices (Osteomy and wound care applications), Carboxymethylcellulose sodium is utilised due to its bioadhesive properties and ability to absorb exudates from applied areas.
Thickening and Binding
Generally, all grades of Carboxymethylcellulose are suitable thickeners and binders. Your product requirements will determine which type of grade is most suitable. The following are just some examples of Carboxymethylcellulose types used in certain formulations:
Stabilizing and Dispersing
The desired consistency of the end product will define the Carboxymethylcellulose grade used as a stabilizer or as a dispersing agent. A low viscous Carboxymethylcellulose will stabilize and disperse, and will not give a significant viscosity increase at concentrations that are already high enough for an effective stabilization. A high viscous type out of the Carboxymethylcellulose grade will stabilize and viscosify the end-product. The structure of the products prepared with highly substituted Carboxymethylcellulose will be somewhat “longer”. It is impossible to list all the formulations, but in general, the following grades are recommended:
Tablet Film Coating
Low molecular weight grades (CEKOL™ 30; and Aqualon™ 7L2P or Blanose™ 7LP EP can be used in tablet coatings, including in combination with sugar solutions. When Carboxymethylcellulose solutions are evaporated, they form strong and flexible films (tensile strength is approx. 500 kg per cm2. The films absorb very little ultraviolet light or wavelengths of 330 nm and larger). Varying with the type of Carboxymethylcellulose yields transparent or almost crystal clear. The films are also resistant to oil and have proven to function well, even at high relative humidity.
Correct steps for Dispersing Carboxymethylcellulose Sodium
Carboxymethylcellulose exhibits good solubility both in cold and hot water. However, it has a tendency to form lumps due to agglomeration caused by rapid swelling. The agglomeration tendency is more pronounced with fine powder than with granular grades.
To avoid agglomeration when adding Carboxymethylcellulose to water, it is necessary to have a good mixing device in order to obtain a quick dispersion of the material’s particles. To achieve a good dispersion, a high-speed mixer should be used and Carboxymethylcellulose slowly added to the vortex formed by the stirrer. Even better is a jet-stream mixer, preferably with an inductor. Besides these methods there are some shortcuts to improve dispersion:
Carboxymethylcellulose may be pre-mixed with another solid ingredient in the formulation, which separates the gum particles and prevents agglomeration and lumping.
Carboxymethylcellulose can be pre-wetted with a non-solvent that is soluble in water, e.g. Ethanol, Glycerine, or Propylethylene glycol, or dispersed in a non-solvent that will be part of an emulsified final product, e.g. edible oils. The effect of the non-solvent is basically the same as in the previous case, i.e. the particles are separated from each other. If a volatile non-solvent is used this can easily be removed from the solution by heating.
Carboxymethylcellulose sodium is incompatible with strongly Xanthan gum.
Carboxymethylcellulose sodium is made from wood cellulose, a naturally occurring, nontoxic, and non-irritant material. Carboxymethylated sodium has been used in pharmaceutical, cosmetics, and food products for several decades, and is generally considered a safe and non-hazardous substance. It is not absorbed to a significant extent following oral update, and its safety in food is well established.
Health authorities consider Carboxymethyl cellulose sodium safe for humans and all animal species, and while over-consumption may cause laxative effects, its use is of no concern for consumer safety. This has been affirmed through numerous reports by various health authorities (e.g JECFA, SCF and EFSA) over the years and allocated an acceptable daily intake (ADI) of ‘not specified’.
The most recent comprehensive evaluation of Carboxymethyl cellulose sodium as a food additive was in 2017 by the EFSA, which concluded that there was no need to set a numerical ADI.
Toxicology: LD50 (rat, oral): 27g/kg
Although hygroscopic under high humidity conditions, Carboxymethylcellulose sodium is generally a stable material (shelf-life 24-36 months). Poorly stored, the material can take up a large quantity of moisture from the environment. In formulated products, this can lead to instability and product deterioration. When constituted as aqueous solutions, Carboxymethylcellulose is stable over a wide pH range (pH 2-10), but extremes of pH are best avoided for optimal stability. To prevent microbial deterioration, a suitable preservative may be added.
During the processing of bulk Carboxymethylcellulose sodium, observance of SHEQ protocols is recommended, including the use of gloves, dust masks, and eye protection so as to minimise contact with mucous membranes and skin. Refer to the MSDS on TWA for your region.
A sustainability score for Carboxymethylcellulose sodium has not yet been provided.
The main grades supplied under the Aqualon™ brand are:
| Grade | Concentration (% w/w) | Viscosity range (mPa s) at 25 C |
| Aqualon™ 7L2P | 4 | 200-200 |
| Aqualon™ 7LF PH | 2 | 25-50 |
| Aqualon™ 7MF PH
Aqualon™ 9M8F PH Aqualon™ 7M8F PH |
2 | 400-800 |
| Aqualon™ 12M31P | 2 | 800-3100 |
| Aqualon™ 9M31F PH | 2 | 1500-3100 |
| Aqualon™ 7HOF PH
Aqualon™ 7H3SF PH |
1 | 1000-2800 |
| Aqualon™ 7HF PH | 1 | 1500-3000 |
The main grades supplied under the Blanose™ brand are:
| Grade | Concentration (% w/w) | Viscosity range (mPa s) at 25 C |
| Blanose™ 7LP EP | 2 | 25-50 |
| Blanose™ 7M1F PH | 2 | 50-100 |
| Blanose™ 7MF PH | 2 | 400-600 |
| Blanose™ 12M8P | 2 | 400-800 |
| Blanose™ 9M20F PH | 2 | 200-800 |
| Blanose™ 7M85F PH
Blanose™ 9M31F PH Blanose™ 12M31P |
1 | 1500-3100 |
| Blanose™ 7HF PH | 1 | 1500-2500 |
| Blanose™ HOF PH
Blanose™ H3SF PH |
1 | 100-2800 |
| Blanose™ 7H4XF PH
Blanose™ 9H4HXF PH |
1 | 2500-4500 |
Nouryon (ex-CP Kelco)
Main Sodium carboxymethylcellulose grades supplied under the CEKOL® brand
| Grade | Concentration (% w/w) | Viscosity range (mPa s) at 25 C |
| CEKOL® 30 | 4 | 200-500 |
| CEKOL® 150 | 4 | 500-2500 |
| CEKOL® 300 | 2 | 200-400 |
| CEKOL® 700 | 2 | 400-1000 |
| CEKOL® 30000 | 1 | 1500-4500 |
| CEKOL® 50000 | 1 | 4500-9000 |
[5] S.K. Niazi, Approved Excipients in Uncompressed Solid Dosage Forms, Handbook of Pharmaceutical Manufacturing Formulations, CRC Press2016, pp. 226-249.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.