Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Dibasic Calcium Phosphate Anhydrous (also known as anhydrous Calcium hydrogen phosphate, among other names) is an inorganic mineral excipient composed of Calcium ions (Ca2+) and phosphate anions in a 1:1 molar ratio. It is supplied as a white, odourless, tasteless powder or crystalline solid. It occurs as triclinic crystals.
Synonyms and Trade Names: Synonyms and Trade Names: Anhydrous Calcium Hydrogen Phosphate; Anhydrous Dibasic Calcium Phosphate; Calcium Hydrogen Phosphate Anhydrous; Anhydrous Dibasic Calcium Phosphate; A-TAB; Calcium Monohydrogen Phosphate; Calcium Orthophosphate; Di-Cafos AN; Dicalcium Orthophospahate; E341; EMCOMPRESS®; Anhydrous Fujicalin®
Pharmacopoeial Compliance: USP-NF; Ph.Eur; IP; J.P; FCC
Uses and Applications: Tablet and Capsule Diluent and Source of Calcium

Dibasic calcium phosphate anhydrous is an inorganic calcium phosphate mineral compound. The Calcium ions (Ca2+) and phosphate anions are present in a 1:1 molar ratio. In the literature, Dibasic calcium phosphate anhydrous is known by many names and acronyms, including Anhydrous calcium hydrogen phosphate, Anhydrous dibasic calcium phosphate; Calcium hydrogen phosphate anhydrous, DCP, DCPA; MCPA, Anhydrous dibasic calcium phosphate, Calcium monohydrogen phosphate, Calcium orthophosphate, and Dicalcium orthophosphate.
Dibasic calcium phosphate belongs to a family of eleven mineral compounds that find important applications in medicine, geology, construction, and dentistry. Their methods of production, function, and applications depend on their structure, composition, solubility, and chemical stability. These related basic properties have been extensively studied; the literature is vast and interdisciplinary. In the pharmaceutical field, the three most important minerals from this family are Dibasic calcium phosphate anhydrous, Dibasic calcium phosphate dihydrate, and Tricalcium phosphate, which are used as diluents and fillers in solid dosage development.
Dibasic calcium phosphate anhydrous has the formula CaHPO4 and has no water of hydration. The “di” prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. When intended to be used as a pharmaceutical excipient, Dibasic calcium phosphate anhydrous is available as a white, odourless, tasteless powder or crystalline solid. It occurs as triclinic crystals.
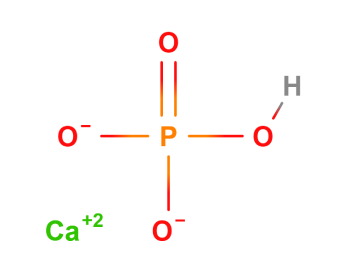
| Chemical Name | Dibasic calcium phosphate |
| CAS Registry Number | [7757-93-9] |
| Empirical Formula | CaHPO4 |
| Molecular Weight | 136.06 |
| EC Number | 231-826-1 |
| UNII Code (FDA) | L11K75P92J |
Dibasic calcium phosphate anhydrous is an approved excipient that is listed in the USP-NF, Ph.Eur and J.P. It is also GRAS listed and included in the FDA Inactive Ingredients Database (oral capsules and tablets).. It is also accepted as a food additive in Europe (E341).
| Form | Solid, powder |
| Appearance | White, odourless powder or crystalline material |
| pH | pH = 5.0 – 7.5 (20% slurry) |
| Angle of repose | 330 |
| Density | 2.89 g/ml |
| Bulk density | 0.85 g/ml |
| Tapped density | 0.45 g/ml |
| Melting point | N/A
Material decomposes at 425oC to form calcium pyrophosphate. |
| Moisture content | Typically 0.1 – 0.2%. The anhydrous material contains only surface-adsorbed moisture and cannot be rehydrated to form the dihydrate. |
| Particle size distribution | Average particle size varies depending on grade/supplier |
| Solubility | Practically insoluble in ether, ethanol, and water; soluble in dilute acids. |
| Specific surface area | 20-45 m2/g |
| Test | USP-NF | Ph.Eur | J.P |
| Official name | Anhydrous Dibasic Calcium Phosphate | Calcium Hydrogen Phosphate Anhydrous | Anhydrous Dibasic Calcium Phosphate |
| Authorised use | Excipient
Source of Calcium |
Anhydrous Dibasic Calcium Phosphate | Anhydrous Dibasic Calcium Phosphate |
| Definition | specified | specified | specified |
| Appearance | White or colourless crystalline powder | White or colourless crystalline powder | White or colourless crystalline powder |
| Identification | specified | specified | specified |
| Acid insoluble substances | specified | specified | specified |
| Carbonates | specified | specified | specified |
| Barium | specified | specified | specified |
| Chlorides | ≤0.25% | ≤0.25% | ≤0.248% |
| Fluorides | ≤0.005% | ≤100ppm | n/a |
| Sulphates | ≤0.5% | ≤0.5% | ≤0.200% |
| Heavy Metals | ≤0.003% | ≤40 ppm | ≤31 ppm |
| Loss of Drying | n/a | n/a | ≤1.0 |
| Arsenic | ≤3 µg/g | ≤10 ppm | ≤2 ppm |
| Iron | ≤400ppm | ≤400ppm | ≤400ppm |
| Assay | 98.0 – 103.0% | 98.0 – 103.0% | ≥98.0% |
| Labelling | specified |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Dibasic calcium phosphate anhydrous is principally a tablet and capsule diluent. It is also used as a source of calcium and phosphorous in nutritional/dietary supplement tablets, where it is one of the most commonly used materials in this role, only second to Calcium carbonate. Specialty grades of Anhydrous dibasic calcium phosphate are also used in toothpaste and dentifrice formulations due to their abrasiveness.
As a pharmaceutical excipient, anhydrous dibasic calcium phosphate is selected for its compaction properties and flowability. The main deformation mechanism (of anhydrous dibasic calcium phosphate coarse-grade) during compaction is brittle fracture, which helps reduce the strain-rate sensitivity of the material, thus allowing an easier scale-up during new product development. Finally, Anhydrous dibasic calcium phosphate is nonhygroscopic and highly stable material that does not absorb water to transform into the dihydrate.
However, unlike the dihydrate, Anhydrous dibasic calcium phosphate can exhibit lamination and capping when the formulations are compressed at high pressures, particularly in formulations where the excipient is present in high proportions relative to the active ingredient, or when deep concave punches are in use. This can be reduced by introducing Microcrystalline cellulose or other suitable dry binders.
When using Anhydrous dibasic calcium phosphate, a high level of lubrication is required since the material is highly abrasive. Typical lubricant levels are >1% w/w, either Magnesium stearate or Sodium stearyl fumarate.
Commercially, Anhydrous dibasic calcium phosphate is available in two grades: a milled, poorly flowing grade, for use with wet-granulation or roller-compression processing, and a coarse, freely-flowing grade material aimed for direct compression.
Anhydrous calcium phosphate finds wide use in pharmaceutical products, food products, and toothpastes owing to its acceptability as a relatively nontoxic and non-irritant material.
Dibasic calcium phosphate anhydrous is a nonhygroscopic and relatively stable material. Under conditions of high humidity it does not hydrate to form the dihydrate. However, the bulk material should be stored in a well-closed container in a dry place.
Workers handling Dibasic calcium phosphate must observe SHEQ precautions appropriate to the circumstances and quantity of material being processed. The fine-milled grades carry the risk of generating dust, thus the use of a respirator or dust mask is advised.
Dibasic calcium phosphate is a naturally-occurring mineral although it may be obtained obtained synthetically using widely available non-critical resources. Being inert and non-toxic, it is considered safe for the environment, with minimal long-term impact on ecology or marine life. Dibasic calcium phosphate achieved a total score of 78/100 by the Excipients Forum Sustainable Chemistry™ assessment scheme.
[2] P.C. Schmidt, R. Herzog, Calcium phosphates in pharmaceutical tableting. 2. Comparison of tableting properties, Pharmacy World & Science : PWS, 15 (1993) 116-122.
[5] C.M. Hentzschel, A. Sakmann, C.S. Leopold, Comparison of traditional and novel tableting excipients: physical and compaction properties, Pharm Dev Technol, 17 (2012) 649-653.
[7] G.K. Reynolds, J.I. Campbell, R.J. Roberts, A compressibility based model for predicting the tensile strength of directly compressed pharmaceutical powder mixtures, International Journal of Pharmaceutics, 531 (2017) 215-224.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.