Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Carrageenan is a high molecular weight (> 100 kDa) sulfated polysaccharide, obtained from red seaweed. The USP – NF describes carrageenan as consisting mainly of potassium, sodium, calcium, magnesium, and ammonium sulfate esters of galactose and 3,6-anhydrogalactose copolymers. Three types of carrageenans are characterised: λ- (lambda) carrageenan; ι -(iota) carrageenan (iota-carrageenan); and K- (kappa-carrageenan). It is supplied as a white or yellow-to-brown coloured powder material devoid of any perceptible odour or taste.
Synonyms and Trade Names: Carrageenan; Chondrus extract; Irish Moss Extract; Marine Colloids; E407; GENUVISCO®, Grindsted® Carrageenan; Hygum® TP; SeaSpen® PF; Viscarin®
Pharmacopoeial Compliance: USP-NF; JPE
Uses and Applications: Emulsifying Agent; Gelling Agent; Stabilizing Agent; Suspending Agent; Sustained-release Agent, and as a Viscosity-increasing Agent

Carrageenan is the common name for several closely related high-molecular-weight (>100 kDa) hydrophilic sulphated polysaccharides. They ALL consist of repeating disaccharide residues of sulfated and non-sulfated galactose and 3,6-anhydro-galactose, linked by alternate units of α-1,3 and β-1,4-glycosidic bonds. They contain between 15% and 40% of ester-sulphate, which makes Carrageenans are anionic polysaccharides.
Carrageenan can be classified into six product families based on their sulphate content, extraction, and solubility, namely: kappa (κ-), iota (ι-), lambda (λ-), mu (µ-), nu (ν-), beta (β-), and Theta (θ-) carrageenan. However, the most important in the pharmaceutical field are kappa-carrageenan (kappa-CG), iota-carrageenan (iota-CG), and lambda-carrageenan (lambda-CG).
In general:
Carrageenan has been used in the human diet for several millennia. References to seaweed and its use in food preparation date back to 600 BC. In the 1300s, seaweed use expanded in Ireland (where it was known as Irish Moss) where it was used to prepare jellies and dairy products. However, it was not until the 1800s that the use of seaweed grew and seaweed farming developed into a major business.
The 20th century saw the addition of further applications, namely stabilisation of ice cream and chocolate milk, meat processing and several fast-moving consumer goods. In 1991, Carrageenan was officially approved by the US FDA as a food additive, followed by the European Union in 1998. Today, the use of carrageenan within the food and non-food sectors is well established. It provides unique solutions for a broad range of products and applications and is frequently the preferred hydrocolloid for functional and cost considerations.
Carrageenan is supplied as a is a yellow-brown to white coloured, coarse to fine powder that is odourless and tasteless.
Carrageenan is a large polymeric molecule consisting of thousands of galactose units. The possibility for structural variations can therefore not be unexpected. The three main types of Carrageenan can be presented as idealized linear polymers with assigned definitive repeating structures. However, natural Carrageenan polymer, like any other natural and complex material, contains many galactose units that are inconsistent with the repeating dimeric structure.
An idealised chemical structure of Kappa-Carrageenan is shown below:
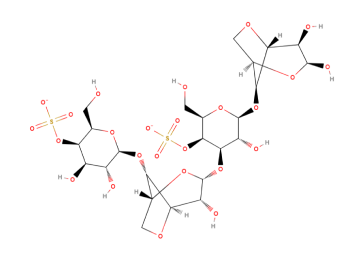
| Chemical Formula | C23H23FN4O7ZN |
| Molecular Weight | >100 kDa |
| Chemical name and CAS Registry Number
Carrageenan ι-Carragecnan k-Carrageenan λ-Carrageenan |
[9000-07-1] [9062-07-1] [11114-20-8] [9064-57-7] |
| EC Number | 232-524-2 |
| UNII Code | 5C69YCD2YJ |
Many properties of Carrageenan depend very much on the type, source, and processing conditions. The following properties apply only to GENUVISCO® Carrageenan CG-130 (CP Kelco):
| Physical form | Solid, powder |
| Appearance | White, yellowish, light grey or light brownish |
| Odour | Not perceptible |
| Flowability | Freely flowing powder |
| Particle size | 100/200 mesh |
| pH (1.0% solution) | 8.0-11.0 |
| Moisture | 12% (maximum) |
| Viscosity (1.5% w/v solution) | 50-80 cP |
| Gel strength (g/cm2) | 100-1200 |
| Solubility | Carrageenan exhibits the solubility characteristics typical of hydrophilic hydrocolloids. Thus, it is soluble in water and insoluble in most organic solvents. Solubility in water is highly influenced by type of Carrageenan, ionic composition of the water, pH and temperature |
| USP-NF | |
| Official name | Carrageenan |
| Authorised use | Excipient |
| Definition | specified |
| Appearance | White or almost white, granular powder |
| Identification | specified |
| Arsenic | 3ppm |
| Heavy Metals | ≤0.004% |
| Lead | ≤0.001% |
| Acid Insoluble Matter | 2.0% |
| Viscosity | ≥5mPa |
| Loss on Drying | ≤12.5 |
| Total Ash | ≤35% |
| Assay | n/a |
| Labelling | Specified |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Carrageenan was initially used as a thickening aid in the food industry, and also due to its gelling, emulsifying, and stabilizing properties. These functionalities have now been adopted in a wide range of fields, including pharmaceuticals, and personal care.
In the pharmaceutical field, Carrageenans are highly versatile materials that can be used to create various textures, ranging from free-flowing liquids to solid gels. The specific uses to which different Carrageenan types are employed derive from their unique gelling properties. Thus:
In this respect, Carrageenan has been used as an emulsifying agent, gelling agent, stabilising agent, thickener and suspending agent, sustained-release agent, and viscosity-increasing agent in oral and topical dosage forms. It is widely used to increase viscosity and aid stability of suspensions (liquid and dry), emulsions, gels, creams, lotions, drops, suppositories, lozenges and gummies. In suspension formulations, usually it’s the iota-carrageenan and lamba-carrageenan that are selected. Generally, the recommended levels are 0.2-0.8% w/v.
Carrageenan’s slipperiness has been advantageously used to improve the mouthfeel of antacid suspensions. Similarly, Carrageenan stabilises emulsions and improves the lubricity of topical products, such as lotions and creams, when used in this range.
ι-Carrageenan develops a shear-thinning thixotropic gel. When used this way, the presence of calcium ions is required for the gel network to become established.
However, the realisation of Carrageenan’s desirable properties requires proper dispersion. Preferably, Carrageenan should be dispersed in cold water, and then heated above its dissolution temperature to obtain optimum functionality. Fine mesh powder increases the powder-liquid interfacial area, which reduces dispersibility and improves the solubility of Carrageenan. A general guide on solubility of different grades is shown in the table below:
| Medium | Kappa | Iota | Lambda |
| Hot water | Soluble above 60 oC | Soluble above 60 oC | Soluble |
| Cold water | Sodium salt soluble
Potassium and calcium salt, insoluble |
Sodium salt soluble
Calcium salts give thixotropic dispersions |
Soluble |
| Concentrated sugar solutions | Soluble hot | Not readily soluble | Soluble hot |
| Concentrated salt solutions | Insoluble | Soluble hot | Soluble hot |
While it is possible to disperse Carrageenan in cold water with high-speed mixing, however, this method can make the particles stick together forming a slowly dissolving sticky film layer around each Carrageenan particle. This leads to the formation of large agglomerates, the so-called “fish eyes”. These “fish eyes” are then very difficult to solubilise due to the protective film layer. What manufacturers advise is that the Carrageenan is preblended with other ingredients, such as sugar at a ratio of 1:10. This keeps the carrageenan particles apart and ensures proper dispersion.
For more tips on usage, you can download this resource by CP Kelco: Guidelines for Proper Dissolution of Carrageenan.
Carrageenan is a common food additive and has been used safely throughout the world for decades. It is approved by the FDA as GRAS and is also considered safe for the general population by the WHO Joint Expert Committee on Food Additive (JECFA) and the European Food Safety Authority. Carrageenan has been tested for safety in various animal models for many years and to date, it has been recognized as safe based on a history of safe use, various acute toxicology studies, and chronic toxicology tests. The WHO has set an acceptable daily intake of carrageenan of ‘not specified’ as the total daily intake was not considered to represent a hazard to health.
Toxicology: LD50 (rat, oral): >5000 mg/kg
Carrageenan is overall a stable, non-reactive substance even though it is moderately hygroscopic (equilibrium moisture uptake 12%). Therefore, it should be stored in a cool, dry place, away from light, heat or moisture. The shelf-life is given as 36 months if storage conditions are complied with.
Carrageenan solutions are quite stable at neutral or alkaline pHs. At lower pHs stability decreases, especially at high temperatures. As the pH is lowered hydrolysis of the Carrageenan polymer occurs, resulting in loss of viscosity and gelling capability. Once the gel is formed, even at low pHs (3.5 to 4.0), hydrolysis is not seen, and the gel remains stable. For practical applications, the processing of carrageenan solutions at low pHs and high temperatures for a prolonged period of time should therefore be avoided.
When handling Carrageenan in an industrial setting, observance of recommended SHEQ protocols is highly recommended. Workers should wear appropriate PPE that fits the circumstances and quantity of material being processed. Consults provided Safety Data Sheets for more information.
Carrageenan is a natural material sourced sustainably from seaweed. It is a biodegradable substance that’s considered safe for the environment with no long-term impact on ecology or marine life. Carrageenan excipient grade achieved a total score of 84/100 by the Excipients Forum Sustainable Chemistry™ Score.
CP Kelco is the #1 producer of Carrageenan, with plants in Europe, the United States, and the Philippines. Grades are marketed under the following brand names:
[1] M. Hariharan, T.A. Wheatley, J.C. Price, Controlled-release tablet matrices from carrageenans: compression and dissolution studies, Pharmaceutical Development and Technology, 2 (1997) 383-393.
[2] K.M. Picker, Matrix tablets of carrageenans. I. A compaction study, Drug Development and Industrial Pharmacy, 25 (1999) 329-337.
[3] V.K. Gupta, M. Hariharan, T.A. Wheatley, J.C. Price, Controlled-release tablets from carrageenans: effect of formulation, storage and dissolution factors, European Journal of Pharmaceutics and Biopharmaceutics, 51 (2001) 241-248.
[4] M. Thommes, P. Kleinebudde, Use of κ-carrageenan as alternative pelletisation aid to microcrystalline cellulose in extrusion/spheronisation. I. Influence of type and fraction of filler, European Journal of Pharmaceutics and Biopharmaceutics, 63 (2006) 59-67.
[5] S. Prajapati, L. Patel, M.A. Patel, Carrageenan: a naturally occurring routinely used excipient, (2007).
[6] H. Kranz, K. Jürgens, M. Pinier, J. Siepmann, Drug release from MCC-and carrageenan-based pellets: experiment and theory, European journal of Pharmaceutics and Biopharmaceutics, 73 (2009) 302-309.
[7] L. Li, R. Ni, Y. Shao, S. Mao, Carrageenan and its applications in drug delivery, Carbohydrate polymers, 103 (2014) 1-11.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.