Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Anhydrous Lactose is a grade of lactose consisting of β-lactose (O-β-D-galactopyranosyl-(1→4)-β-D-glucopyranose) and/or a mixture of β-lactose and α-lactose (O-β-D-galactopyranosyl-(1→4)-α-D-glucopyranose). It is supplied as a white, or creamy white, hard crystalline material, which is readily milled into a powder. It is odourless, has a slightly sweet taste.
Pharmacopoeial Compliance: USP-NF; Ph.Eur; BP; JP
Synonyms and Trade Names: Anhydrous Lactose; Anhydrous 60M; Lactopress Anhydrous; SuperTab 22AN; Anhydrous Impalpable; Lactopress Anbydrous; Lactopress Anhydrous 250; SuperTab 21AN; SuperTab 22AN
Uses and Applications: Directly Compressible Tablet Filler and Roller Compression Diluent
Anhydrous lactose exists as either the α-lactose or β-lactose that is without its water of hydration, although the α-form is most often used in pharmaceutical applications. It is described by official pharmacopoeia either as O-β-D-galactopyranosyl-(1,4)-β-D-glucopyranose or a mixture of O-β-D-galactopyranosyl-(1,4)-α-D-glucopyranose and O-β-D-galactopyranosyl-(1,4)-β-D-glucopyranose. It is supplied as white to off-white crystalline powder.
Lactose (IUPAC name of lactose is 4-O-(β-d-galactopyranosyl)-D-glucopyranose) is a naturally occurring disaccharide that consists of one molecule of β-D-galactose and one molecule of β-D-glucose molecules linked through a β1-4 glycosidic linkage. It is produced by the mammary epithelium of all lactating mammals. To date, milk is the only known significant source of lactose.
For pharmaceutical applications, Lactose is produced from whey as a by-product of the dairy industry. The process involves the crystallisation of a saturated whey concentrate. To minimise risk from contamination from Bovine Spongiform Encephalopathy (BSE), additional refining and purification steps are undertaken, the result of a which are a chemically pure excipient that carries no risks arising from being an animal derived raw material.
Lactose exists naturally in the form of two isomers: α-Lactose (i.e β-D-galactopyranosyl-(1,4)-α-D-glucopyranose) and β-lactose (i.e β-D-galactopyranosyl-(1,4)-β-D-glucopyranose). The two forms have specific optical rotations [α]20D of +89.4o and +35o, respectively, dur to their differences in the spatial positioning of a H-atom and the -OH-group on C1 in the glucose moiety.
Conversion between the two anomers occurs via the open chain form of the glucose moiety, and depending on the concentration and temperature, an equilibrium will establish at [α]20D +55.3o, corresponding to ≈37% α-Lactose, and 63% β-Lactose. Changes in concentration or temperature can shift the equilibrium accordingly (for example, an increase in temperature or concentration increases levels of β-Lactose, and vice versa). At typical conditions, however, Lactose in solution is considered to be a mixture of α-, and β-Lactose.
Lactose crystallises from solution when its equilibrium solubility is exceeded (for instance, through the removal of water or a lowering of temperature). Various Lactose crystal forms can theoretically form. When Lactose is crystallised under standard processing conditions (typically <93.5 oC), the main crystalline form obtained is the α-Lactose monohydrate form, which is also the most stable form. α-Lactose monohydrate forms exist as hard and brittle ‘tomahawk-like’ crystals and is not hygroscopic. In the hydrated form, this form α-Lactose contains one mole of lactose and one mole of water. The water of hydration can be removed if the α-Lactose is heated to above 140 oC which produces anhydrous α-Lactose.
If the crystallisation is conducted at >93.5 oC, anhydrous β-lactose is obtained. This anomer forms small, kite-like brittle crystals that are brittle but also markedly more soluble. It exhibits minimal hygroscopicity, however, it is unstable, and will transform back to the α-Lactose form given the right conditions.
When a solution of Lactose is spray-dried, the rate of water removal is too rapid for crystallisation to occur. In this case, amorphous Lactose is produced which exists in a glassy state. This form also contains some water of hydration. Amorphous Lactose is hygroscopic and can adsorb water causing crystallisation into the α-Lactose monohydrate as a result of molecular mobility.
As a result of the differences in the physicochemical attributes of the different forms of lactose, grades of Lactose exhibit differences in parameters such as melting point, density, and solubility, and ultimately, in their functionalities when it comes to their uses as pharmaceutical excipients.
Commercially, there are several different brands of anhydrous lactose which contain anhydrous α-lactose and anhydrous β-lactose. It does not have water in its crystal lattice. Typically, however, the levels of anhydrous β-lactose range between 70 and 80% while anhydrous α-lactose ranges between 20-and 30%. It is milled and sieved to permit grading into desired particle size distributions and to optimise flowability and compactibility.
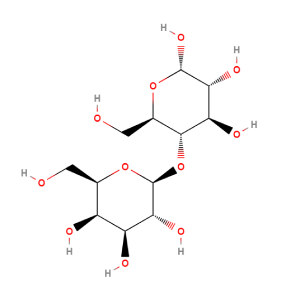
| IUPAC Name | O-β-D-galactopyranosyl-(1→4)-β-D-glucopyranose |
| CAS Registration Number | [63-42-3] |
| Empirical Formula | C12H22O11 |
| EC/EINECS Number | 200-559-2 |
| UNII Code (FDA) | 3SY5LH9PMK |
Anhydrous lactose is an approved pharmaceutical excipient and is listed in all the major pharmacopoeia. It is also GRAS listed and included in the US FDA Inactive Ingredients Database (injections, oral tablets and capsules and inhalation). A specification for lactose is included in the Food Chemicals Codex FCC.
| Physical form | Solid (powder) |
| Appearance | White to off-white crystalline powder |
| Flowability | Medium to High |
| Compactibility | High |
| Brittle fracture index | 0.0362 (at compression pressure 177.8 MPa) |
| Bonding index | 0.0049 (at compression pressure 177.8 MPa) |
| Permanent deformation pressure | 521.0 MPa (at compression pressure 177.8 MPa) |
| Tensile strength | 2.577 MPa (at compression pressure 177.8MPa) |
| Reduced modulus of elasticity | 5315 (at compression pressure 177.8 MPa) |
| Bulk density | 0.71 g/cm3 (SuperTab 21AN) |
| Tapped density | 0.88g/cm3for SuperTable 21AN |
| True density | l.589 g/cm3 for anhydrous β-lactose |
| Melting point | 232.00 C (average) |
| Particle size distribution | D10 = 10 µm, D50 = 130 µm and D90 340 µm (DuraLac® H – Meggle) |
| Solubility | Soluble in water; sparingly soluble in ethanol |
| Specific rotation | 54.4o to 55.9o |
| Test | USP-NF | Ph.Eur |
| Official name | Anhydrous Lactosee | Lactose Anhydrous |
| Authorised use | Excipient | specified |
| Definition | specified | specified |
| Identification | A
B C |
A
B C D |
| Characters | n/a | White or almost white crystalline powder |
| Acidity of alkalinity | specified | specified |
| Appearance of solution | n/a | specified |
| Specific optical rotation | 54.4o – 55.9o | 54.4o – 55.9o |
| Absorbance
210-220nm 270-300nm 400nm |
≤0.25 ≤0.07 ≤0.04 |
≤0.25 ≤0.07 ≤0.04 |
| Heavy Metals | ≤5 µg/g | ≤5ppm |
| Water | ≤4.5 – 5.5% | ≤4.5 – 5.5% |
| Sulphated ash | ≤0.1% | |
| Microbial contamination
Aerobic bacteria Fungi and yeast Absence of E.coli & Salmonella |
100cfu/g 50cfu/g specified |
100cfu/g 50cfu/g specified |
| Protein and light-absorbing impurities | specified | n/a |
| Loss on drying | ≤0.5% | n/a |
| Content of alpha and beta anomers | specified | specified |
| Assay | n/a | n/a |
| Labelling | specified | n/a |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Anhydrous lactose is a versatile raw material that can be used as an excipient in tablet and capsule formulations, as a carrier in dry powder inhalers, and as a lyophilization agent in parenteral (biotechnology-type) productions. For the most part, however, it is used in solid dosage forms, mainly in direct compression owing to its brittle nature, which allows pre-compaction without loss of function. Indeed, Anhydrous lactose offers the best compactibility of all available grades of lactose, and was developed in order to meet the unique demands of direct compression operations. These requirements are met through the careful control of process parameters during production, notably the particle size as well the ratio of beta anhydrous lactose and alpha lactose monohydrate.
Anhydrous lactose yields strong compacts when utilised in direct compression tableting applications. It may also be used in wet granulation applications although its may advantages are realised during roller compaction, where it outclasses popular excipients such as Microcrystalline cellulose since it does not experience precompression loss of compatibility. and as a tablet and capsule filler and binder. Anhydrous lactose can be used with moisture-sensitive drugs due to its low moisture content. It may also be used in intravenous injections.
Lactose is widely used in oral pharmaceutical products and may also occasionally be used in intravenous injections. It has been used in pharmaceutical tablets for more than 100 years, thus its safety and toxicity are not in question. Furthermore, pharmaceutical grades of Lactose are required to achieve very high purity levels, including those standards required by the different monographs of the various pharmacopoeia.
Upon ingestion, Lactose is not actively absorbed by the intestine. For absorption to occur, the Lactose molecule needs to be hydrolysed into glucose and galactose. In vivo, the enzyme, lactase, produced by GI tract epithelial cells are responsible for splitting lactose. In many mammals, the activity of lactase wanes shortly after weaning and then remains constant. However, in some individuals, the level of lactase is very low or non-existent. These individuals are not able to digest lactose (malabsorbers). Lactose that remains in the intestine undigested is transferred into the large intestine where it is fermented by gut flora to produce organic acids, such as lactic acid. This can cause additional symptoms linked to water retention, such as bloating, diarrhoea, and abdominal cramps.
The complete limit on sugar in diets of diabetic patients is no longer the conventional approach. The aim of treatment is to achieve and maintain normal blood glucose levels. This can be accomplished by following a normal diet as long as energy intake is controlled and spread evenly throughout the day. For a diet consisting of between 150 and 250g of carbohydrates, the contribution to this by the amount of lactose ingested through tablets is insignificant. Thus, restrictions for diabetic patients to avoid lactose-containing medicines are not warranted.
Lactose in its non-hydrolysed form is minimally cariogenic compared to sucrose. Streptococcus species that break down carbohydrates to produce organic acids responsible for enamel erosion are much less capable of digesting lactose compared with sucrose. Furthermore, since tablets and capsules are commonly swallowed with water, the rinsing effect leaves little residue for bacteria to work upon.
In the past, there base been concerns over the transmissible spongiform encephalopathies (TSE) contamination of animal-derived products. However, in the light of current scientific knowledge, and irrespective of geographical origin, milk, and milk derivatives are reported as unlikely to present any risk of TSF contamination; TSE risk is negligible if the calf rennet is produced in accordance with regulations.
Toxicology: LD50 (rat, IP): > 10g/kg; LD50 (rat, oral): > 10g/kg; LD50 (rat, SC): >5g/kg
Lactose is overall a highly stable excipient when stored under ambient conditions. It is also relatively inert from a chemical point of view and shows minimal tendency to react with formulation ingredients, including active ingredients or other excipients. However, mould growth may occur under humid conditions (80% RH and above). For this reason, the raw materials should be stored correctly in accordance with recommended storage conditions of the manufacturer. it is especially important for Anhydrous lactose to be stored in a well-closed container in a cool, dry place. Observe normal precautions appropriate to the circumstances and quantity of material handled. Excessive generation of dust, or inhalation of dust, should be avoided.
Lactose is a natural disaccharide consisting of galactose and glucose and is present in the milk of most mammals. Commercially, it is produced from the whey of cows’ milk; whey being the residual liquid of the milk following cheese and casein production. Cows’ milk contains 4.4 – 5.2% lactose; while lactose constitutes 38% of the total solid comment of milk. A naturally-derived substance, lactose is an inert and non-toxic excipient and considered safe for the environment, with minimal long-term impact on ecology or marine life. However, while the dairy industry has faced questions about its long-term sustainability, lactose is a secondary product of the dairy sector and therefore represents a net realisable value. Lactose Monohydrate excipient grade achieved a total score of 72/100 by the Excipients Forum Sustainable Chemistry Score™
Kerry Group (Sheffield Biosciences)
[2] H. Ando, S. Watanabe, T. Ohwaki, Y. Miyake, Crystallization of Excipients in Tablets, Journal of Pharmaceutical Sciences, 74 (1985) 128-131.
[4] M.D.C.M. Perales, A. Muñoz‐Ruiz, M.V.V. Antequera, M.R.J.C. Ballesteros, Study of the Compaction Mechanisms of Lactose‐based Direct Compression Excipients using Indentation Hardness and Heckel Plots, Journal of Pharmacy and Pharmacology, 46 (1994) 177-181.
[5] L.E. Flores, R.L. Arellano, J.J.D. Esquivel, Study of Load Capacity of Avicel PH-200 and Cellactose, Two Direct Compression Excipients, Using Experimental Design, Drug Development and Industrial Pharmacy, 26 (2000) 465-469.
[7] L. Farber, G.I. Tardos, J.N. Michaels, Evolution and structure of drying material bridges of pharmaceutical excipients: studies on a microscope slide, Chemical Engineering Science, 58 (2003) 4515-4525.
[8] K.M. Nagel, G.E. Peck, Investigating the Effects of Excipients on the Powder Flow Characteristics of Theophylline Anhydrous Powder Formulations, Drug Development and Industrial Pharmacy, 29 (2003) 277-287.
[9] H. Takeuchi, S. Nagira, M. Aikawa, H. Yamamoto, Y. Kawashima, Effect of lubrication on the compaction properties of pharmaceutical excipients as measured by die wall pressure, Journal of Drug Delivery Science and Technology, 15 (2005) 177-182.
[10] G.K. Bolhuis, N. Anthony Armstrong, Excipients for direct compaction—an update, Pharmaceutical Development and Technology, 11 (2006) 111-124.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.