A product’s label is a window in its soul, that’s what the product stands for, its benefits to its consumers. Claims such as ‘Natural’, ‘Fresh’ or ‘Artificial’ have a particular resonance with consumers, playing a key role in shaping purchase decisions and other behaviours.
Within the personal care and food/nutrition sectors, ‘natural’ status carries a lot of acclaim, as evidenced from the ever increasing sales of natural products or those with ‘natural’ claims. Natural skincare and nutrition brands have been growing at twice the rate of conventional products, a trend product developers and marketers seem to know very well about.
In the pharmaceutical sector, natural claims, while not carrying similar implications, are still important. Not only are there many excipients and active ingredients, including carbohydrate polymers, gums, surfactants, oils, flavours, alkaloids and antibiotics that are natural in origins or are naturally derived but consumers everywhere are increasingly seeking natural products due to their perception as being sustainable and friendly to the planet.
However, despite the widespread use of the term natural, the definitions of the terminology around natural, natural origin or artificial remains, to this day, in the regulatory equivalent of ‘no man’s land’. Regulators are as yet to provide official definitions or guidance on what is natural or not. Maybe because of the legal ambiguity, consumer groups and lawyers have been pushing for restrictions and/or legal redress on the uses of ‘natural’ and claims around ‘no artificial’ ingredients.
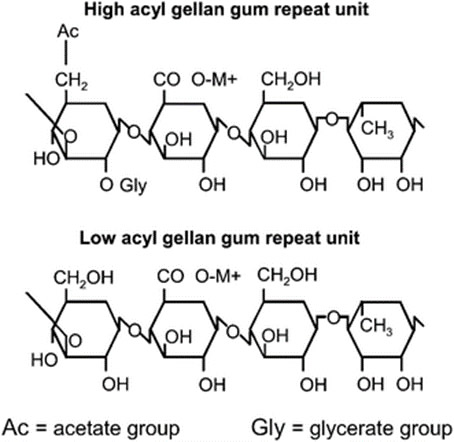
Consider xanthan gum, a gum that is widely used in the food, personal care and pharmaceutical products. Xanthan gum is produced by the bacterium, Xanthomonas campestris, which converts glucose into the gum. The process is a ‘natural’ fermentation process that occurs at its own pace without human intervention. Yet controversy as to whether xanthan gum is a natural product or not persists. Manufacturers using xanthan gum and attaching ‘natural’ claims to it have been a target of lawsuits challenging the ‘natural’ status of this material.
So what do ‘natural’ and ‘naturally derived’ mean?
This is by no means a definitive, legal guide. The FDA has not provided a regulatory definition of what natural entails when it comes to regulated products. The EU has guidelines on the use of ‘natural’ for nutritional claims (Regulation (EC) 1925/2006 but when it comes to other product groups, there still remains ambiguity. Therefore the use of the term ‘natural’ in advertising or labelling requires good judgment and legal counsel to avoid legal jeopardy.
Nevertheless, a material is considered ‘natural’ when it is ordinarily identified in nature, obtained and used in its natural, raw state after being extracted from the source. For example, Virgin Almond Oil Ph. Eur is the oil obtained by cold expression of the ripe seeds of Prunus dulcis. No further treatment (refining) or additives have been added.
The types of ‘permitted’ treatments include physical, enzymatic or microbiological processes on the plant or animal source material.
A material is referred to as ‘naturally derived’ or ‘natural origin’ when the natural source is treated to access other properties of the natural raw material; for example, getting natural fatty acids and natural fatty alcohols from a coconut.
By way of an example, natural fatty acids may require further processing in order to unlock or create certain aspects of the material’s properties beneficial for its function. Equally, potassium benzoate and lecithin require extraction and purification before they are in a state suitable for performance as preservatives or emulsifiers. Provided they are from plants, these materials can be classified as naturally derived.
A less encountered term is ‘nature identical’ which is typically used in relation to flavours. Nature identical flavours (vs artificial flavours) are flavours obtained by synthesis or isolated through chemical processes. The components are chemically and organoleptically identical to flavouring components present naturally present in nature. From a regulatory perspective, nature identical and artificial flavours are undistinguishable.
Standards and Certifications
A number of international standards and certification schemes on naturalness have been developed in recent times. They aim to help raw material suppliers and product manufacturers harmonise definitions on ingredient definitions and guide consumers. Although initially aimed at the cosmetics sector, the technical definitions and qualification schemes are equally applicable to food and pharmaceutical ingredients.
Below are three of the most important standards and schemes:
The COSMOS-standard
The COSMOS-standard was introduced in 2010. It aims to define and implement a standard for organic and natural cosmetics. The COSMOS standard includes guidelines on origin and approved processing procedures of the ingredients if they are to qualify as natural or organic. In addition to ingredients, the COSMOS standard also defines naturalness of the total product.
NaTrue Standard
The NaTrue Standard was developed by the NaTrue Scientific Committee Criteria and Label, an international non-profit organization. Its main focus is on cosmetics for which it seeks to clarify and promote natural and organic cosmetics globally. For ingredients, the NaTrue Standard differentiates between natural substances, nature-identical substances and derived natural substances, with a list of approved treatment processes. Furthermore, there are positive lists available for nature-identical substances, comprising pigments, minerals and preservatives.
ISO 16128
The ISO 16128 is a standard from the Geneva-based International Standard Organization. It aims to provide “Guidelines on technical definitions and criteria for natural and organic cosmetic ingredients and products”. ISO 16128 consists of two parts. Part 1 provides definitions for different ingredient categories. Part 2 describes the procedure on how to calculate the naturality of ingredients and the natural origin content of a cosmetic formulation, based on the amount of natural components in each raw material.
Conclusions
Even with the availability of standards and certifications the distinction between natural and unnatural is not always clear. In the absence of regulatory guidance, it falls to the formulator and marketer to make their own decision about what is natural and what is not, as there is no absolute definition, only opinions. This technical note will hopefully assist you in navigating the maze of natural and artificial ingredients.