Economic Outlook for Pharma in 2022
Throughout 2020 and 2021, navigating the pandemic has been an all-consuming endeavour for all sectors. As the pandemic raged, pharma responded with leadership and purpose, managing to deliver a vaccine in record time. However, with the looming energy crisis, supply chain crunch and labour market tightening all coming to a head, the question on most people’s minds is what does the economy hold out for pharma in 2022 and beyond?
Economic picture
Last month, leading economists at the World Bank, the International Monetary Fund, the OECD and the Conference Board released global economic growth forecasts for 2022 through to 2026.
World growth for 2022 is forecast at 3.9%; with growth across all mature economies forecast to be up by 3.9%. The US and China, the main engines of global economic growth are expected to grow at 3.8% and 5.5% respectively.
| Real GDP (average annual) % change | Actual | Actual | Actual | Estimate | Forecast | Forecast | Trend |
| 2000-2009 | 2010-2019 | 2020 | 2021 | 2022 | 2023 | 2022-2026 | |
| United States | 1.9 | 2.2 | -3.4 | 5.7 | 3.8 | 3 | 2.1 |
| Europe | 1.7 | 1.7 | -6.6 | 5 | 4.1 | 1.7 | 1.2 |
| Euro Area | 1.4 | 1.3 | -6.9 | 4.7 | 3.9 | 1.5 | 1 |
| United Kingdom | 1.6 | 2 | -9.7 | 7 | 4.5 | 1.4 | 1.1 |
| Japan | 0.4 | 1.2 | -4.7 | 2.5 | 3.3 | 1.4 | 0.8 |
| All mature economies | 1.8 | 2 | -4.6 | 5.1 | 3.9 | 2.3 | 1.7 |
| China | 8.9 | 6.3 | 2.2 | 5 | 3.3 | 3.2 | 3.7 |
| India | 6.8 | 7.2 | -7.1 | 7.5 | 8.5 | 4.3 | 4 |
| Brazil | 3.4 | 1.4 | -4.4 | 5.2 | 1.5 | 1.7 | 1.6 |
| Russia | 5.4 | 2 | -2.9 | 4.5 | 2.1 | 1.8 | 1.6 |
| Turkey | 3.9 | 5.8 | 1.6 | 8.9 | 2.5 | 3.3 | 3.6 |
| All emerging econo | 5.7 | 4.7 | -2.1 | 5.2 | 4 | 3.2 | 3.2 |
| World | 3.4 | 3.3 | -3.3 | 5.1 | 3.9 | 2.8 | 2.5 |
The pharmaceuticals market is expected to grow, fuelled by new scientific and operational opportunities, as companies rearrange their operations and recover from COVID-19 , which had earlier led to restrictive containment measures involving social distancing, remote working, and the closure of commercial activities that resulted in operational challenges. The market is expected to reach $1700.97 billion in 2025 at a CAGR of 8%.
Among the upside and downside risks identified in the forecast, global supply chain problems, labour issues, and businesses technology investments pause the greatest risks to growth.
Supply chain
The reason supply chain problems are concerning this time round is that, unlike past disruptions (from natural disasters, international trade tensions, cyberattacks, and even, the global pandemic), the current crises appears much harder to resolve.
There is little companies or a single sector can do when its cargo ships are having to wait to unload in goods in ports. The current crisis is compounded by energy price inflation, and the resulting shortages of critical inputs, such as active pharmaceutical ingredients and excipients. This is why this supply shock has no quick fixes, and a rapid return to business as usual does not seem to be on the horizon, anytime soon.
Labour shortages
Labour shortages are crucial to growth. The economists believe that to a certain extent, workers have become more selective in the kind of work they’re taking on post-pandemic. We think this argument is overly simplified and the reality is far more complex.
Taking the US as a case study, three million more workers retired than were expected to retire during the pandemic. In part, this was because older workers are at greater risk of being harmed by COVID. But many older workers found that their retirement plans, and the value of their homes, increased significantly in value. On the other hand, younger workers have had more choices for work.
To attract the workers needed, companies are having to pay new workers higher wages and offer better benefit plans. As the young go back to work in 2022, the need to do this will decrease.
Demographic issues, in the longer term, will also contribute to a shortage in workers. The citizenry is aging in the established economies as well as China. To overcome this, businesses are increasingly investing in digital technologies.
However, one economist warned, the decade before the pandemic hit had the slowest growth in productivity in many decades. Nonetheless, in certain industries, technology is clearly replacing humans and that the pandemic has helped accelerate this shift.
While supply chain and labour issues will depress growth, it is important to put this in perspective. Global economies will grow significantly faster than is the norm.
You can read the report in its entirety through this link.
Global Economic Outlook 2022: Global GDP growth forecast (conference-board.org)
Additional reporting by Niall P Hughes
Medication Swallowing Difficulties: The 3 Steps Needed To Improve Patient Experience
Dr. E Vickers (with additional contributions from E Mwesigwa) | Pharmacentral.com
Individuals with swallowing difficulties face inequalities in their access to safe medicines and could be at a greater risk of poor health outcomes compared with the general population. This article sets out to highlight the scale of the problem and suggests actions that the pharmaceutical industry and regulators can take to improve the situation.
What are Swallowing Difficulties?
Swallowing, the act by which we ingest solid food, liquids or medication, is in fact a highly intricate process that requires the interplay of several nerves and muscles in the oral cavity, pharynx and oesophagus in order to safely transfer bolus into the stomach.
While the vast majority of us do this simple act without much thought, there are millions of people who, for one reason or another, struggle to swallow. For them, the ability to initiate and complete a normal swallow is tortuous, accompanied by anxiety, pain, choking or aspiration.
Swallowing disorders occur in all age groups, either as a result of congenital abnormalities, damage to structures in the oropharyngeal anatomical structures and or short-term or long-term medical conditions. In some age groups and populations, however, swallowing difficulties are far more significant and pernicious. For example, in children with learning disabilities as well as senior citizens who need daily medication to alleviate their conditions.
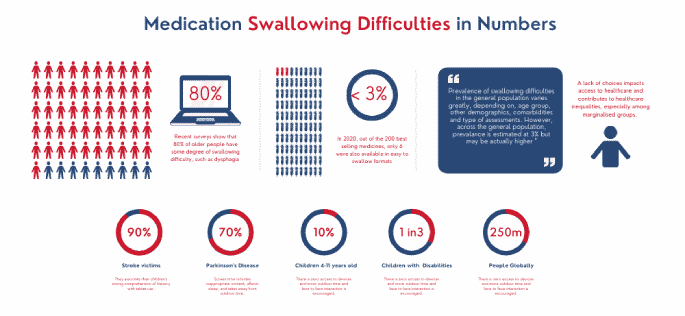
In some situation, an inability to swallow solid medication can be a matter of life and death. For instance, in those with Parkinson’s disease where 70-80% of sufferes have swallowing problems or those who have had a stroke, where swallowing difficulties run at 90%.
Read about our article on Empathy – What the pharmaceutical industry can learn from the IT Industry
The main causes of swallowing difficulties catalogued in the medical literature include:
- Dysphagia, the most well-known among swallowing disorders, refers to a group of disorders characterised by changes in the structures or neurological control of the swallow. Studies show that dysphagia affects 3 % of the general population.
- Odynophagia which refers to pain swallowing caused by irritation or infection of the oral mucosae and oesophagus, particularly in individuals with acquired immunodeficiency syndrome, oesophagitis or disorders of motility of the oesophagus.
- Phagophobia which is the fear and avoidance of swallowing foods, liquids or medication, usually based on the person’s fear of choking. It is on a psychological dimension and characterized by swallowing complaints but no abnormalities upon physical examination or investigation.
Note that difficulty to swallow is not in itself a disease, rather it may be an indication of an underlying structural, neurological or other dysfunction for which proper medicare should be sought since factors that lead to abnormal swallowing, whether it is dysphagia, odynophagia or phagophobia, can be life limiting, and if severe, life threatening.
Anatomy and Physiology of Swallowing
The normal swallow permits an individual to handle a wide range of solid and liquid products of varying volumes, textures and consistencies. This process can generally be divided into different phases, depending on whether the material is a liquid or a solid.
But first, it is essential to quickly review the anatomy and physiology of swallowing as a basis for appreciating swallowing difficulties and how to design effective interventions.
The anatomy of the oral cavity, pharynx, larynx and the innervations of the muscle in the oral cavity are shown in the figure below:
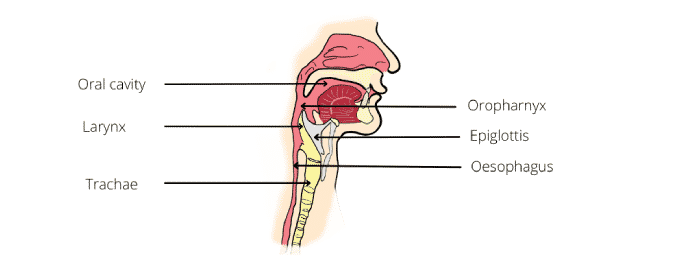
The tongue has both oral and pharyngeal surfaces. The oral cavity is separated from the pharynx by the faucial pillars. The pharynx has a layer of constrictor muscles that originate on the cranium and hyoid bone, and the thyroid cartilage anteriorly.
Note that the anatomy of the head and neck of infants is different from that of adults. In infants, teeth are not erupted, the hard palate is flatter, and the larynx and hyoid bone is higher in he neck to the oral cavity. The epiglottis touches the back of the soft palate so the larynx is open to the nasopharynx, but the airway is separated from the oral cavity by a soft tissue barrier.
The physiology of normal eating and swallowing is described by two models: the four stage model for liquids and the process model for solids.
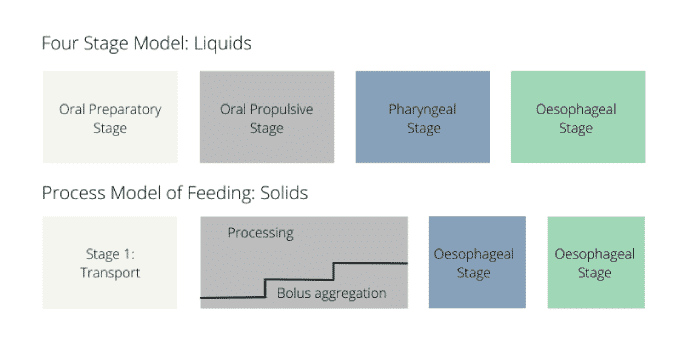
Although there are differences in the sequence of events in the two models, it is possible to reduce the swallow to three main phases as follows:
Oral Phase
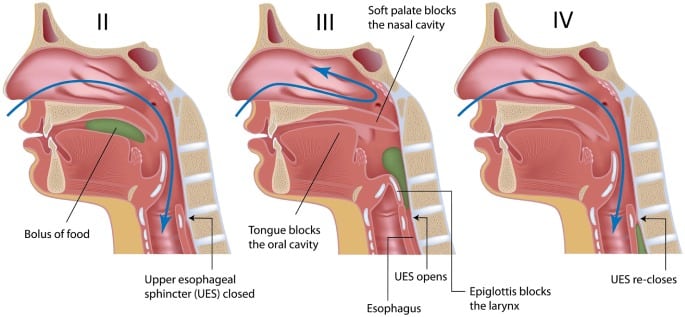
Upon introducing a liquid or solid into the mouth, the material is prepared into a bolus and or transported to the middle of the tongue. During this stage, the posterior part of the oral cavity will be sealed by the action of the soft palate and tongue, thus preventing premature leakage of bolus into oropharynx before the swallow. Note that the tendency for leakage increases with age.
After a brief moment the anterior tongue rises, touching the alveolar ridge of the hard palate just behind the upper teeth. The posterior tongue drops, opening the back of the oral cavity. The surface of the tongue lifts upward, propelling the bolus back along the palate and into the pharynx.
Pharyngeal Phase
The pharyngeal swallow is a swift activity that follows the oral phase. It serves two main purposes:
(1) to permit bolus to be propelled through the pharynx and the upper oesophageal sphincter and into the oesophagus, and
(2) to protect the airway by preventing entry of food into the larynx and trachea.
In this phase, the soft palate elevates and contacts the walls of the pharynx, leading to the closure of the nasopharynx at the point the bolus hurtles into the pharynx. The base of the tongue retracts, pushing the bolus against the pharyngeal walls. Constrictor muscles of the pharynx then contract, squeezing the bolus downward, and together with retraction of the base of the tongue, pushes the bolus downward.
For obvious reasons, the ability to safely pass bolus through the pharynx without aspirating or regurgitation into the nasal cavity is important in human swallowing. Therefore, there are several mechanisms at play which the body uses to prevent entry of food particles into the airway during swallowing.
Oesophageal Phase
The oesophageal phase describes the transport of the bolus through the oesophagus. The oesophagus is a tube-shaped structure originating from the lower part of the upper oesophageal sphincter and terminating at the lower oesophageal sphincter.
During the swallow, the muscles relax allowing the bolus to pass down. Movement is facilitated by a series of peristaltic waves, as well as gravity, both of which effectively transport the bolus through lower oesophageal sphincter and into the stomach.
Swallowing Abnormalities
Abnormal swallowing can result from a wide range of conditions and disorders related to the anatomy and or physiology or the oral, pharyngeal and oesophageal dysfunction.
Swallowing difficulties manifest in different ways, which include:
- Painful chewing or swallowing
- Dry mouth (Xerostomina)
- Difficulty controlling solids or liquids in the mouth
- Hoarse or wet voice quality
- Coughing or chocking before, during or after swallowing
- Feeling of obstruction (globus sensation)
Dysphagia
Dysphagia arises from abnormalities in structure or motility and ranges from inability to initiate swallowing to solids getting stuck in the oesophagus.
Generally, two main types of dysphagia are recognised:
Oropharyngeal dysphagia, whereby patients are unable to transfer food into the oesophagus by swallowing. Oropharyngeal dysphagia is subdivided into structural/obstructive and neurological/propulsive.
From a clinical point of view, any difficulties swallowing solids indicates either structural or propulsive oropharyngeal dysphagia, while difficulty swallowing liquids indicates propulsive or neurological oropharyngeal dysphagia.
Oesophageal dysphagia is when patients can initiate swallowing process however as the food passes down the oesophagus and into the stomach, they experience discomfort. The underlying causes can also be structural or propulsive abnormalities.
The table below lists some of the most common causes of oral and pharyngeal dysphagia
Common Causes of Oral and Pharyngeal Dysphagia
| Neurological disorders and stroke | Structural lesions | Psychiatric disorders |
| Cerebral infarction
Brain-stem infarction Intracranial haemorrhage Parkinson’s disease Multiple sclerosis Motor neurone disease Poliomyelitis Myasthenia gravis Dementias |
Thyromegaly
Forestier’s disease Congenital web Zenker’s diverticulum Ingestion of caustic material Neoplasms |
Psychogenic dysphagia
Polymyositis
Connective tissue diseases: Polymyositis & Muscular Dystrophy
Iatrogenic Causes: Surgical resection Radiation fibrosis Medication |
From: Palmer Jb et al, 2006. In Braddom R (ed): Physical Medicine and Rehabilitation, Elsevier, Philadelphia. Pp 597-616.
Odynophagia
Odynophagia is the disorder in which swallowing is associated with pain. It differs from dysphagia, which is simply difficulty when swallowing — and does not associate with pain, whereas odynophagia always does.
Odynophagia can be caused by infective and non-infective inflammatory processes, benign and malignant esophageal disorders such as achalasia, gastro-esophageal reflux disease and carcinoma.
Some of the conditions associated with odynophagia include:
- Gastroesophageal Reflux Disease
- Esophagitis
- Candidiasis
- Esophageal Cancer
Phagophobia
Phagophobia is a relatively rare type of anxiety disorder associated with swallowing. It is often mixed up with pseudodysphagia, which is the fear of choking. The key difference between these two phobias is that individuals with phagophobia are anxious about the act of swallowing whereas those with pseudophagia are afraid that swallowing will lead to choking.
Irrespective, phagophobia and pseudodysphagia can be life limiting, and in the case of medication, life threatening. This is especially the case in the small but significant cohort of individuals, who for reasons still to be known, have phagophobia and pseudodysphagia related to medication.
Unfortunately, the causes of phagophobia are poorly understood and may even be multifactorial, involve past experiences, underlying health conditions or simply learned through observing others who struggle to swallow certain things.
It has been found that individuals who watch others experience difficulties (e.g pain or embarrassment) when swallowing may go on to develop phagophobia.
Finally, phagophobia may occur in the absence of any underlying triggers.
Symptoms of phagophobia include:
- Anticipatory anxieties before ingestion of meals
- The tendency to eat very small mouthfuls or drinking frequently or large amounts of liquids during meals as a way to aid swallowing
- Extreme anxiety and fear at the thought of swallowing
- Panic attacks
- Rapid heart rate and breathing
- Reluctance or avoidance of eating or drinking in front of others
- Sweating
- Switching to an all-liquid diet as a way to alleviate anxiety around swallowing
- Weight loss (skipping medication and exacerbation of illness if related to medication)
Oral Medicines and Swallowing Difficulties
The prevalence of swallowing difficulties varies greatly, including population under consideration, comorbidities and assessment methods. Experts contend that prevalence may actually be greater than published figures would indicate since many patients may not report symptoms.
Generally, 70 – 90% of all seniors have some degree of swallowing difficulty. In certain cases, for instance, Parkinson’s disease and Stroke, swallowing difficulties are the norm, and have been reported to be as high as 90%. According to a recent study, swallowing difficulties run at 3% in the world adult population, but are 10 times higher in those with neurological and or psychological conditions, such as learning disabilities, severe mental illness or dementia.
With oral administration of medication being the most preferred route, the swallowing of solid medication, particularly tablets and capsules, presents specific challenges to anybody with swallowing problems. To make matters worse, solid dosage forms need to be taken with water, which requires the same individuals to control a thin fluid, which complicates matters even more.
Which medication types are suitable for dysphagia and other swallowing difficulties?
Most medication in use today is formulated as tablet dosage forms. According to the British Pharmacopoeia, a tablet is circular in shape with either a flat or convex faces prepared by compressing the active pharmaceutical ingredients with excipients.
In reality, they are available in a wide range of sizes, shapes, colours and indentations. In addition, tablets may be sugar or polymer film coated as well.
The oral route of drug administration is the most preferred route of taking medicine, and understandably, manufacturers of medicines recognise this. As a result, oral medicines account for more than 70% of all medicines in use.
Tablets (and more specifically, standard compressed tablets) are the single most popular dosage form, responsible for 50% of all pharmaceutical preparations manufactured and sold. Some of the reasons for popularity of tablets include:
- Tablets allow accurate dosage of medicament to be prefabricated and administered simply and conveniently
- Tablets are consistent with respect to weight and appearance
- Drug release rate can be fine-tuned to meet physiological and pharmacological needs of patients
- Tablets can be mass-produced simply and quickly, which allows the wider public to have access to medicines that would otherwise be too costly.
However, to anyone with swallowing difficulties, swallowable tablets are a nightmare. Problems with the neural control or the structures involved in swallowing mean that swallowable tablets are not ideal sufferers of dysphagia. Too big (frankly, most are) and they are a choking hazard. Too small and they become difficult to detect on the tongue and move around in the mouth to initiate a safe swallow.
If the tablets can be crushed beforehand, it can greatly help pass them down however, as with anything that requires precision, the possibility of errors increases with the number of additional manipulations. Thus, having technologies that enable dosing without the need for additional dilution, elaboration or mixing as is always needed in paediatric, geriatric or other swallowing disorders would be of great benefit.
There are alternatives to swallowable tablets, which depending on the type of drug substance and its intended use, may be considered:
- Buccal Tablets
- Caplets and Coated Tablets
- Chewable tablets
- Effervescent Tablets
- Lozenges
- Mintablets
- Multiparticulates
- Orally Disintegrating Tablets (ODTs)
- Powders for reconstitution
- Sublingual Tablets
- Hard Gelatin Capsules
- Soft Gelatin Capsules
- Chewing Gums
- Gummies
- Topical Products (Ointments, Creams, Lotions and Transdermal Patches)
- Parenteral Products
- Inhalation Products
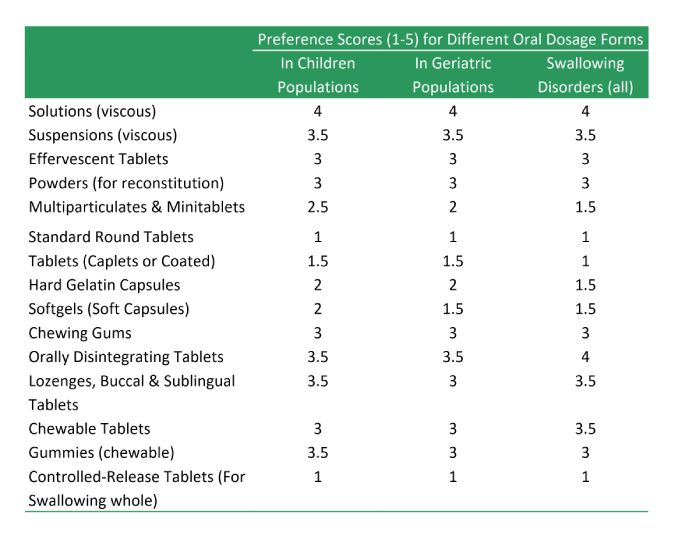
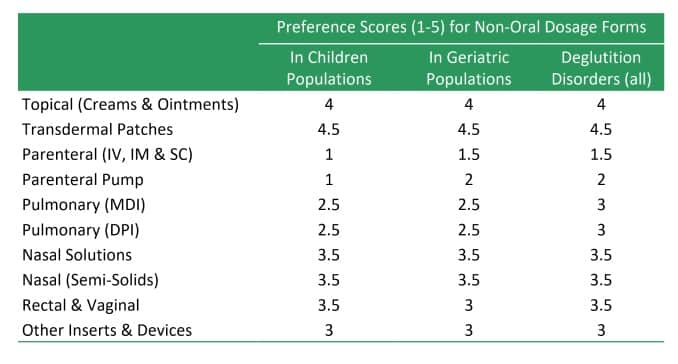
Key Characteristics of Different Tablets Types
| Type of tablets | Description and Advantages | Key Considerations |
|---|---|---|
| Swallow Tablets | The vast majority of tablets fall in this category. These tablets are designed for per-oral administration by swallowing | Most tablets belong to this category. They are designed to be swallowed whole although some may be crushed/split up to ease ingestion. |
| Buccal Tablets | Buccal tablets are designed to be placed under the cheek mucosa or between the lip and gum. Typically designed for slow release and absorption. | Buccal tablets permit administration without the requirement for water/swallowing Drugs should not be bitter or unpleasant in the mouth Usually very small and flat and do not require addition of a disintegrant |
| Sublingual Tablets | Sublingual are designed to be placed under the tongue. Unlike buccal tablets, they allow rapid absorption through blood vessels under the tongue while avoiding 1st-pass effect. | Sublingual tablets permit administration without the requirement for water/swallowing Drugs should be soluble, typically low dose and not be bitter or unpleasant in the mouth Careful selection of excipients required |
| Orally Disintegrating Tablets (ODTs) | ODTs are designed to rapidly disintegrate on the tongue into a smooth solution or suspension that can be swallowed without the need for water. | Two key characteristics that a dosage form labelled as an ODT must possess is a rapid disintegration time of 30 s or less, and a tablet weight of 500 mg or less |
| Chewable Tablets | Chewable tablets consist of a mild effervescent excipient base which can be chewed and broken down into a smooth consistency which can be swallowed. | To provide fast disintegration and dissolution, the tablet should be designed to be soft or easy to chew. The active drug substance must not be unpleasant to the taste, and frequently, flavours and sweeteners are required. |
Unfortunately, too many products on the market today are formulated with little consideration of those with swallowing difficulties. Products for children are perforce prepared from products designed for adults; and the same applies for the elderly, who often have swallowing problems while also requiring prolonged, non-crushable tablets. In 2020, for instance, out of the 200 best-selling medicines in the United States, only six were offered in easy-to-swallow formats. It is not funny any longer. It is unsafe and something needs to be done soon.
That people have to crush medication in the 21st century so that children and seniors can be treated despite the wide availability of technologies and excipients and knowhow is disgraceful.
Actions needed to reduce inequalities in dysphagia
The prescription remains the most widely used medical intervention today. Yet it is estimated that up to 50 % of all patients prescribed medication fail to take it correctly. This not only leads to waste of resources but could lead to treatment failure and sub-optimal outcomes.
If society is to equitably offer quality healthcare to all, it will be necessary to take a whole person approach, by recognising the many root causes of inequality, and engender system-wide action, from regulators, pharmaceutical companies, patient groups as well as healthcare workers as the immediate contact points for patients.
Here is a range of preventative actions that local areas can take to reduce inequalities and improve health outcomes and the lives of people with mental illness.
1. A better understanding of the scale of the problem
Although dysphagia and other swallowing disorders are widespread, the scale of the problem, especially as it relates to medication, is still poorly understood.
It is generally known that patients, for one reason or another, tend to underreport their problems during contact with healthcare providers. The lack of understanding on the scale of the problem generally hinders society’s ability to deliver equitable healthcare.
Therefore, investment in data gathering is urgently required if we are to fully understand the scale of this problem. When delivering care, providers and institutions need to ask patients if they have issues swallowing solid medicines and know the implications of not offering working solutions.
In the 21st century, it is not just a patients’ physical comfort that is important but also their emotional well-being. This way, more joined-up interventions can be implemented.
2. Partnership between the public, government and pharmaceutical companies
With growing healthcare needs, increasing expectations from the health systems, and challenges of insufficient resources, it is unlikely that health services can be provided solely by a single actor. More than ever before, healthcare requires profit and social purpose to converge.
Public-private partnerships (PPPs) have traditionally taken many forms, varying in the level of participation or risk taken by different parties. We are not talking about PPPs as such, but rather, collaborative framework in which patient organizations, the pharmaceutical industry and healthcare providers work together, get closer to patients and gain deeper insights about their individual issues and not just as patients.
Although there is no-one-size-fits-all model, such a collaborative model can actually facilitate development of better therapies.
3. Legislation and incentivisation of marketing authorisation applicants
Providing medicines for marginalised or neglected demographics, such as those with swallowing problems, has been an endemic oversight in the pharmaceutical industry. This has been partly because marginal groups have not always been a viable commercial market or because companies were simply not bothered.
Given how prevalent dysphagia and other swallowing issues are, urgent action is required. There is need to join forces to pressure regulators and drug producers to address this inequality. One way is to require applicants for marketing authorisations to provide introduce alternative formats aimed at those with swallowing difficulties at launch in return for reduced regulatory fees or marketing exclusions.
It is clear that the current strategy of relying on the largesse of individual companies is not working, and a more sustainable approach is required.
Final thoughts
The vast majority of medication available today is in the form of swallowable tablets. These formats are often not appropriate for patients with dysphagia or other swallowing difficulties. Lack of availability of suitable formats for suffers predisposes these groups to sub-optimal treatments and contributes to healthcare inequalities.
If society is to equitably offer quality healthcare to all, it is necessary to take a whole person approach, recognise the many root causes of inequality, and engender system-wide action, from regulators, pharmaceutical companies, patient groups as well as healthcare workers as the immediate contact points for patients.
Sources Used
Overview of Drug Therapy in Older Adults. The Merck Mannual. (available at https://www.msdmanuals.com/en-gb/professional/geriatrics)
Wright, D., 2014. Prescribing Medicines for Patients with Dysphagia. New York: Grosvenor House Publishing, pp.1-101.
Lisa Tews, Jodi Robinson.,2007. Dysphagia. In Kauffman T, L et al., (editors). Geriatric Rehabilitation Manual (Second Edition), Churchill Livingstone, pp 381-385. https://doi.org/10.1016/B978-0-443-10233-2.50063-8. (https://www.sciencedirect.com/science/article/pii/B9780443102332500638)
Dysphagia. National Institute on Deafness and other Communication Disorders. (available at https://www.nidcd.nih.gov/health/dysphagia)
ComCor study: New results on places of infection with SARS-CoV-2 and analysis of the efficacy of messenger RNA vaccines against the Delta variant
WHO update on Omicron
Pharmaceutical Matrix Forming Excipients: Overview, Types, and Selection Criteria
As medical needs become more complex, the need for modified release pharmaceutical dosage forms becomes ever more salient. The matrix tablet technology, which uses matrix forming excipients, has been instrumental in this quest. But exactly what is a matrix former? How do they function, and how are they used? This technical note provides an overview, types and function of this important class of pharmaceutical ingredient.
What is a matrix forming excipient?
Matrix forming excipients are a class of materials used to fabricate sustained-release (prolonged release) pharmaceutical tablets. The matrix former creates a three-dimensional network that not only acts as a structural and functional scaffold for active ingredients but is the physical embodiment of the dosage form.
The matrix controls the entry of GI fluids into the tablet which retards the rate of diffusion of the active ingredient from the tablet, hence, its elimination half-life (time taken for the drug’s plasma concentration to reduce by 50%).
Matrix tablets have been around for more than half a century. Their benefits are easy to see when you consider the fact that 75 percent of all sustained release solid dosage forms used by patients today utilise matrix systems, a proportion that is larger than tablet film coatings, and many other approaches currently available for use to pharmaceutical scientists.
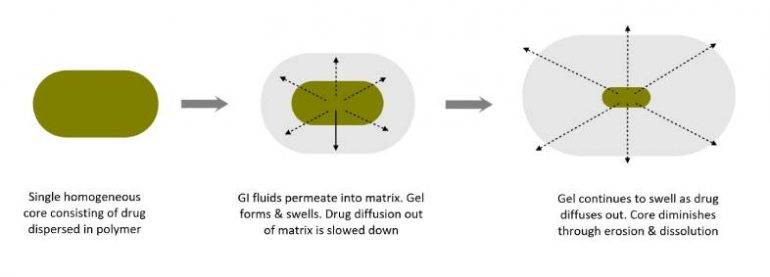
Why are matrix formers used in tablets?
The main reason for using tablet matrices is to achieve sustained release drug delivery. These dosage forms are designed to deliver active ingredients over a period of up to 24 hours. This results into longer-lasting therapeutic effects while reducing dosing frequency and side effects, while improving individual’s well-being.
The achievement of both goals improves patient adherence to medication, making treatments more successful, while also reducing costs of healthcare, especially for chronic conditions. This is increasingly important, due to changing demographics (aging), and children with medical complex needs. Click here to read about the importance of applying empathy in new drug development, and the challenges faced by patients with swallowing problems.
Matrix tablets are selected to formulate sustained release oral dosage forms because of ease and the economics of their manufacture. These products can be easily manufactured by direct compression or wet granulation depending on the type of polymer and additional excipients used in the formulation.
Generally, active drug substances best suited for incorporation into matrix systems exhibit the following characteristics:
- They exhibit neither very slow nor very fast rates of absorption and elimination. Drugs with slow rates of absorption or elimination half lives in excess of 10 hours are inherently self-retarding.
- Drug substances that have very short half-lives below 2 hours require special care during development because of potential huge fluctuations in plasms levels
- Drug substances whose solubility in aqueous media should be good and also maintain adequate residence time in the gastrointestinal tract.
- Active ingredients that are poorly absorbed or at unpredictable rates are also not good candidates for sustained release formulations.
- Finally, drug substances with very narrow therapeutic windows or really small doses are also poor candidates for sustained release formulations because of the difficulty of limiting control of release and preventing dose dumping.
Like what you're reading? Please share it to your network.
Types of matrix forming excipients
The different materials currently used to formulate matrix tablets can be classified into four groups on the basis of the basis of their drug retarding mechanism:
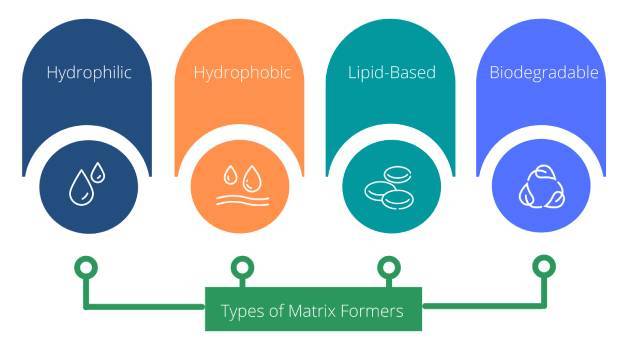
Hydrophobic matrix formers
Hydrophobic matrix materials are water-insoluble large molecular weight polymers for use in the fabrication of sustained release dosage forms. Examples of hydrophobic matrix formers include
- Polyethylene
- Polyvinyl chloride
- Ethylcellulose, and
- Acrylate copolymers
Sustained drug release is produced as a result of swelling and diffusion through a network of channels that exist between compacted polymer particles. The rate-controlling step in these formulations is speed of liquid entry into the matrix.
Lipid matrix formers
Lipid-based matrix formers are high melting point fatty acids and/or waxes capable of forming matrices for sustained release. Drug release from such matrices occurs through both pore diffusion and erosion.
Release characteristics are therefore more sensitive to digestive fluid composition than to purely insoluble polymer matrices.
Examples of lipid matrix formers include
- Carnauba wax in combination with stearic acid,
- Glyceryl dibehenate
- Glyceryl tristearate
- Tripalmitin
- Trymyristin, and
- Hard Fats
Lipid matrices are inert, non-eroding and non-dissolving systems that achieve drug release prolongation through the creation of a hydrophobic domains in the tablet. These domains not only slow down the hydration of the tablet but they also control the rate of dissolution and release of the drug into the aqueous milieu.
Hydrophilic matrix formers
Hydrophilic matrix formers are the most widely used materials of their kind owing to their ease of use, cost-effectiveness and wider regulatory acceptance. They are typically hydrophilic, high molecular weight polymers with high gelling capacities. Upon contact with water, they swell and gel, creating a moving barrier that regulated the rate of drug release. For this reason, they are also known as swelling controlled release systems.
Polymers used in the preparation of hydrophilic matrices are divided in to three broad groups
- Cellulose derivatives, such as methylcellulose, hydroxypropylcellulose, Hypromellose, and Sodium carboxymethylcellulose.
- Non cellulose natural or semi synthetic polymers such as Agar-Agar; Carob gum; Alginates; Xanthan gum, Pectin, Chitosan and Modified starches.
- Polymers of acrylic acid, such as Carbomer 934
Biodegradable matrix
The last category of matric forming materials are a special group of polymers that are biologically degraded or eroded by enzymes to generate simple metabolites that are eliminated through the usual processes. These polymers may be natural polymers such as proteins and polysaccharides, semi-synthetic polymers or fully synthetic systems, such as the well-known aliphatic poly (esters) and poly anhydrides.
Read about the key differences between Hypromellose and Polyethylene Oxide matrix forming excipients here:
Hypromellose v Polyethylene Oxide for the Formulation of Matrix Mini Tablets
Examples of drug products that use matrix former technology
Here are examples of tablets formulated as matrix systems for controlled release:
Theophylline
Theophylline is a methylxanthine, chemically similar to caffeine. It is one of the drugs used in the treatment of asthma and COPD due to its safety and low cost. Theophylline sustained release tablets use hydrophilic polymers such as hydroxyethylcellulose and hypromellose in combination with polyethylene glycol 6000 and cetostearyl alcohol (as a dissolution rate retardant).
Paliperidone
Paliperidone, which is also referred to as 9-hydroxyrisperidone, is the primary active metabolite of risperidone. It is a widely prescribed antipsychotic medication today. The oral dosage forms are available as sustained release tablets using polyethylene oxide and hydroxyethylecellulose as the matrix forming excipients.
Oxycodone
Oxycodone is a semisynthetic opioid drug substance derived from thebaine. It is similar to hydrocodone and morphine. It is currently used for moderate to severe pain due to its powerful analgesic and sedative properties. It exhibits excellent oral bioavailability and is commonly used and/or co-formulated with other analgesics. It is available as sustained release tablets, immediate release tablets, oral solutions and injectables. Matrix tablets when used as the drug release prolonging technology make use of ammoniomethacrylate copolymer (Eudragit® RS 30D), polyvinyl acetate and Glyceryl dibehenate.
Felodipine
Felodipine is a methyl and ethyl diester of 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid. Together with amlodipine and nifedipine, felodipine is a calcium-channel blocker that lowers blood pressure by reducing peripheral vascular resistance through a highly selective action on smooth muscle in arteriolar resistance vessels.
Felodipine sustained release tablets are variously formulated using either hydrophilic or hydrophobic matrix formers, such as Hypromellose K4M and hydrogenated castor oil.
Benefits of using matrix forming excipients
The reasons why particular matrix formers are selected for use by pharmaceutical formulation scientists are many and varied. There is no doubt, however, that as a technology class, these materials offer many advantages, which are outlined below:
- Improve patient convenience and compliance by enabling the formulation of prolonged release dosage forms (e.g once-a-day tablets) that reduce dosing frequency
- Highly versatile in terms of the active ingredients that can be formulated and the manufacturing methods
- Enable the reduction of drug toxicity since the decrease plasma fluctuations and maintain drug within the therapeutic window
- They can enhance product stability
- Prolonged release drugs permit the use of lower amount of total active ingredients thereby reducing drug utilization and exposure
- Improves bioavailability of some drugs
- Matrix formers are relatively inexpensive as a technology
Summary about pharmaceutical matrix formers
Matrix tablets are widely used in the development of sustained release products. A wide range of inactive ingredients known as matrix formers, including hydrophilic, hydrophobic and biodegradable polymers, as well as high melting point lipids are used in matrix tablets. These systems are popular because they are well studied and are amenable for manufacture on standard equipment and processes.
Sources
Pharmacentral has a strict referencing policy and only uses peer-reviewed studies and reputable academic sources. We avoid use of personal anecdotes and opinions to ensure the content we present is accurate and reliable
- Wise, D. L. (2000). Handbook of Pharmaceutical Controlled Release Technology. New York, Marcel Dekker.
- Spiepmann J, Peppas NA (2001). Modelling of Drug Release from Delivery Systems Based on Hydroxypropyl Methylcellulose (HPMC). Adv. Drug Deliv. Rev. 48: 139-157. DOI: 1016/s0169-409x(01)00112-0. Pubchem Google Scholar
- Shargel L, Wu-Pong S, Yu ABC (2012). Applied Biopharmaceutics and Pharmacokinetics, 6th ed., McGraw-Hill Companies.
Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New York City managed to attach a pig kidney to a human patient. The kidney functioned normally for 54 hours.
Last week, a team of surgeons in New York City were to able to successfully attach a pig kidney to a human patient and watch the organ function normally for a whole 54 hours. While procedures of this kind are not new in nonhuman primates, it is the first time that a pig kidney has been transplanted into humans and not been immediately rejected.
The process of transplanting living cells, tissues or organs from one species into another is what scientists call xenotransplantation. However, owing to genetic differences between species, past xenotransplantation efforts have not been successful, leading to immediate organ rejection by the human immune system.
The breakthrough procedure, which was announced at a news conference and widely reported in the media on October 21, represents a giant step towards the aim of increasing availability of life-saving organs for transplantation. Waiting list for donated organs around the world are in the millions, and demand is not expected to drop anytime soon despite a rise in organ donation registrations.
Speaking on condition of anonymity, a nephrologist at Abbott Northwestern Hospital, Minneapolis, MN said the fact that transplant survived three days with full function and no signs of rejection was an “incredible achievement,” and gives fresh confidence that “patients will have access to additional sources of organ for transplantation in the near future”.
However, several years of more of research, clinical trials and regulatory scrutiny are required before we can start to see pig kidneys on surgical tables.
In a press release, Robert Montgomery, MD and chair of the department of surgery at NYU Langone and director of the NYU Langone Transplant Institute, noted that the future of this work is not limited to kidneys.
“Transplanting hearts from a genetically engineered pig may be the next big milestone,” he said. “This is an extraordinary moment that should be celebrated — not as the end of the road, but the beginning. There is more work to do to make xenotransplantation an everyday reality.”
Why pigs?
In the quest to address the chronic shortage of organs, scientists have long sought the use animal organs. Pigs have emerged as an interesting choice because their organs are anatomically similar to those of humans, and they can be easily bred in a highly controlled manner.
However, it is more that ease of breeding. In the mid-20th century, xenotransplantation scientists noticed that transplanted animal organs quickly turned black, a phenomenon known as hyperacute rejection.
As knowledge has improved, scientists have been able to use genetic engineering to overcome some of these challenges. For example, it was found that aggressive immune responses seen after a pig xenotransplant was due to antibodies detecting alpha-gal, a sugar moiety found on porcine vasculature.
Disabling the gene that codes for alpha-gal was key to addressing hyperacute rejection of pig organs. Until now, a test of this sort of transplant hadn’t been done successfully in humans.
So what did the New York University Langone Health team do?
In order to overcome the many ethical hurdles of performing such an operations in humans the surgical team approached the family of a woman with brain stem death kept alive on a ventilator. Although the woman was an organ donor, her organs were not suitable for donation.
Over a period of several hours, the surgical team worked to attach the pig kidney, which had been genetically engineered to remove the alpha-gal sugar to blood vessels, in the upper leg of the patient. The kidney was kept outside of the body so the team could assess its function in real time.
In order to improve chances of acceptance, the team also transplanted the animal’s thymus gland, which aids the education of the immune system to recognize the kidney as part of the body. The patient was also given specific drugs that suppress the immune system.
Within minutes, the kidney started producing large amounts of urine and showed other signs of normal functioning. The pig kidney functioned just like a human kidney transplant. The research team stopped monitoring at 54 hours in line with IRB ethical guidance.
The patient was taken off life support after the procedure.
What’s next for these sort of transplants?
To survive 54 hours represents a significant development but to become mainstream, animal kidneys will need to survive for years not days. For this to happen, researchers will need to show that these organs can withstand immune system attacks for years in the human body.
A key aspect of this journal is to show that transplants are safe in the long-term and obtain approval from health authorities, including the U.S. FDA and the European Medicines Agency.
Are there any ethical concerns about breeding pigs for organ harvesting?
Using any animal for the sole benefit of humans raises important ethical questions. Advocates for xenotransplantation argue the potential benefits of expanding the organ supply are worth any potential harm done to animals.
The jury is still out on how acceptable the idea of breeding millions of pigs in order to harvest organs for human transplantation is.
PETA (People for the Ethical Treatment of Animals), a campaigning organisation against the use of animals in research, contests the whole idea that we should consign animals as sources of spare parts for humans (see their statement through this link).
Questions or comments on this article? E-mail us at editor@pharmacentral.com.
Sources
NYU Langone Health. Progress in xenotransplantation opens door to new supply of critically needed organs. Published online October 21, 2021.
Pharmaceutical Suspending Agents: Overview, Types, and Selection Criteria
Suspending agents are excipients added to disperse systems in order to maintain particulate ingredients in suspension or to prevent other forms of physical instability. In this technical note, we provide an introductory review of what suspending agents are, and outline the function they play in pharmaceutical formulations.
Definition of a Pharmaceutical Suspending Agent
Pharmaceutical suspending agents are a class of excipients added to disperse systems to ensure that any solid particles in the formulation are kept uniformly distributed within the continuous phase, thereby maintaining physical stability of the product.
Pharmaceutical dispersions include suspensions, creams and aerosols. They typically comprise dispersions of insoluble solids (drug and/or excipients) in an aqueous or non-aqueous liquid medium, known as the continuous phase.
Depending on the particle size of the disperse phase, disperse systems can be further categorised as:
- Colloidal (the dispersed particles are in the submicron range), and
- Coarse (dispersed particles are above colloidal size).
Note that the term ‘suspending agent’ is frequently used interchangeably with another, closely related term, ‘thickening agent’.
As we will see, while having some commonalities the two excipients groups are different. Very simply, thickeneing agents can be used as suspending agents but not all suspending agents are thickening agents.
This is best illustrated by the following commonly used OTC products:
Pepto Bismol® is an oral suspension of bismuth salicylate (active). It is formulated with aluminium magnesium silicate, methylcellulose, and gellan gum, which are the materials used to hold the active ingredient in suspension by increasing viscosity (thickeners).
Gaviscon® is an oral suspension of sodium bicarbonate, calcium carbonate, and sodium alginate. The sodium alginate functions both as a suspending excipient and active ingredient.
Calpol® is an oral suspension of paracetamol formulated with maltitol and sorbitol (syrups), microcrystalline cellulose, sodium carboxymethylcellulose, and xanthan gum, as the suspending agents. Suspension of the active is achieved through thickening of the dispersing phased.
In order to appreciate the utility of suspending agents, it’s necessary to quickly review the technical aspects of pharmaceutical disperse systems.
The process of dispersing solid particles in a liquid creates several problems, including:
- Creaming
- Sedimentation
- Flocculation
- Caking
- Particle growth, and
- Adhesion onto the walls of the container (in some formulations)
The above phenomenon constitute physical instabilities, and are generally dependent on three main factors:
- Differences in density between the dispersed particles and the dispersion medium (∆ρ)
- Particle size (radius) of the dispersed phase r
- Viscosity of the dispersion medium (η)
The interrelationship between these three factors is given by Stokes’ law, which is shown below:
v=2gΔρr²/9η
where v is the rate of particle settling (or creaming, for that matter).
According to Stokes law, instability can be reduced by reducing particle size, decreasing density differences between the two phases or increasing the viscosity of the continuous phase.
It is worth knowing that particles will still be able to move about in the dispersion, however, the rate and intensity of collisions will be minimised. Furthermore, disperse systems are highly dynamic – dispersed particles frequently contact each other as a result of the following phenomenon:
- Brownian motion
- Creaming
- Sedimentation due to gravitational forces
- Convection
Given enough time, and in the absence of any other mitigating circumstances, the dispersed particles eventually come into close contact and may coalesce, form bonds and cake, destroying the balance of the disperse system.
Whether dispersed particles reach this point of no return depends on two factors:
- Forces of attraction or repulsion, and
- Nature of the surface of the dispersed particle
This is where suspending agents come into play, i.e they help minimise dispersed particle flux (sedimentation, creaming or convection) or interfere with particle-particle attraction.
We can therefore define pharmaceutical suspending agents as a specific category of excipients added to disperse systems to minimise disperse phase coalescence and instability.
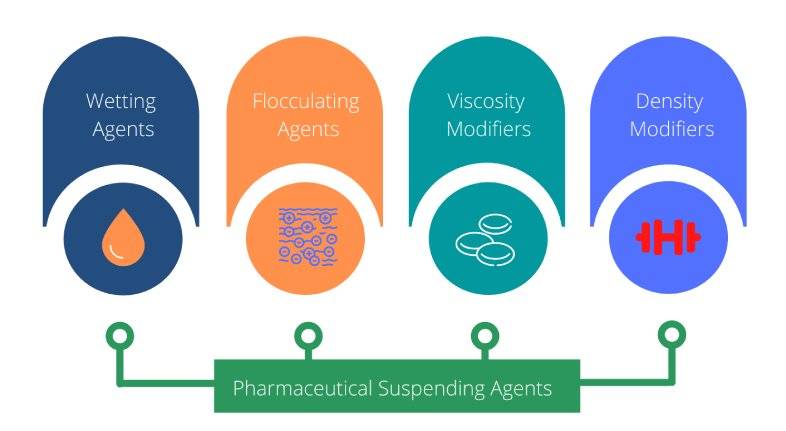
Pharmaceutical suspending agents can be grouped into four main categories as outlined below:
- Wetting agents
- Flocculating agents
- Viscosity modifiers (thickeners)
- Density modifiers
Types of Pharmaceutical Suspending Agents
A). Wetting agents
For the disperse phase to freely and distribute in the continuous phase, it needs to be wetted by the liquid. The presence of entrapped air pockets on the particle surface or if the particles are hydrophobic is a hindrance to dispersion.
Wettability can be enhanced through the reduction of the interfacial tensions, namely solid-liquid and liquid-vapour interfaces.
1. Surface active agents
Surface active agents (surfactants for short) are excipients that lower the surface tension (or interfacial tension) between two liquids or between a liquid and a solid.
Surfactants with HLB values between 7 and 9 have been shown to be suitable wetting agents in pharmaceutical disperse systems.
This is because the apolar groups of the surfactant are able to adsorb onto hydrophobic parts of formulation particle while the polar groups project into the aqueous medium, resulting in a lowering of the interfacial tension between the solid and the liquid.
2. Hydrophilic colloids
Hydrophilic colloids are high molecular polysaccharide polymers or clays. They include acacia, bentonite, tragacanth, alginates and cellulose derivatives.
Being hydrophilic, they are able to coat particles to impart hydrophilic character to the disperse phase. In addition, hydrophilic colloids may also increase viscosity of the continuous phase, reducing the rate of sedimentation of particles.
3. Solvents
Common polar pharmaceutical solvents such as glycerol, propylene glycol and polyethylene glycol and alcohol are highly water-miscible and are able to reduce the liquid-air interfacial tension.
They enable water to infiltrate deep into power agglomerates, displacing entrapped air and enabling wetting of the dispersion to take place.
B). Flocculants
A well-formulated disperse system is one that exhibits the correct degree of flocculation. Systems that are underflocculated generally tend to settle and sediment very rapidly although the compacts formed are loose and redispersion is possible.
On the other hand, overflocculation results in products that while settle slowly the sediments pack tightly such that redispersion is not possible.
By controlling the degree of flocculation, it’s possible to reach a happy medium – in which there is a degree of flocculation and also deflocculation.
This can be achieved through, not only through particle size control and viscosity-modification, but also by using flocculating agents, including electrolytes, ionic surfactants and polymer flocculating agents.
1. Electrolytes
The addition of inorganic electrolytes to the solution changes the zeta potential of the dispersed particles, and provided this is lowered sufficiently enough, will produce a flocculated system.
2. Surfactants
Ionic surfactants can also be used to create flocculation in a disperse system by neutralising particle charges and creating a deflocculated system.
3. Polymeric flocculants
Several polymeric excipients are capable of forming gel-like networks in disperse systems thereby adsorbing onto and trapping dispersed particles and holding them in a flocculated state.
These materials are mainly hydrocolloids although not always, and include:
C). Viscosity modifiers
The deal disperse system is one that displays pseudoplastic or thixotropy behaviour, that is, exhibit high apparent viscosity at low shear rates.
Upon storage, the dispersed particles either settle slowly or, and preferably, remain suspended. When the product is sheared, for instance, when shaken by the consumer, the high apparent viscosity of the formulation should fall sufficiently for product to be dispensed easily.
The various materials currently used as viscosity modifiers are outlined below:
1. Polysaccharides
Examples of polysaccharide-based viscosity modifying excipients include the following:
2. Water-soluble cellulose ethers
Several cellulose ethers have the ability to increase viscosity of aqueous systems in which they are dispersed. They include:
- Methylcellulose
- Hydroxyethylcellulose
- Sodium carboxymethylcellulose, and
- Microcrystalline cellulose
- Hypromellose
The viscosity-increasing properties of cellulose ethers depends on the molecular weight and degree of substitution.
3. Hydrated silicates
Hydrated silicates are naturally-occurring siliceous clays that exist as colloids in water. This group of pharmaceutical grade clays includes:
- Bentonite
- Hectorite
- Magnesium aluminium silicate (VEEGUM®)
Hydrated silicate materials exhibit thixotropic behaviour at low concentration in water and as gels at high concentration.
4. Carbomers
Carbomers are high molecular weight cross-linked polyacrylic acid polymers that swell in water to form viscous hydrogels depending on the degree of cross-linking.
Among carbomer excipients, carbomer 940 is the most commonly used suspending excipient in both topical and oral products.
If you would like to learn more about carbomers, here is a link to an article that gives an overview on these important excipients:
D). Density modifiers
From probing Stokes’ law above, it is clear that if the densities dispersed and dispersing medium are of the same magnitude sedimentation would be significantly slowed down.
Thus, changing the density of the dispersing medium, for example, addition of glycerol, propylene glycol, polyethylene glycol or sucrose-based syrups, can significantly modify densities and leveraged to control instability.
You read more about viscosity modifying excipients through this link:
Summary of Pharmaceutical Suspending Agents
Many pharmaceutical products are formulated as disperse systems in which particulate solids (active ingredients and/or excipients) are distributed throughout a continuous or disperse phase. Owing to the tendency towards settling or creaming by the disperse phase, the use of suspending agents is mandated.
There are many different types of pharmaceutical suspending agents; they can be grouped into four main classes depending on the mechanism of function:
- Wetting agents
- Flocculating/deflocculating agents
- Viscosity modifiers
- Density modifiers
Careful selection of suspending agents is essential in order to have a product that ensures the disperse phase does not settle rapidly, the particle do not settle into a cake, the system is easily re-dispersed into a uniform mixture when shaken, is easy to dispense from the container or use by the consumer.
Sources and Further Reading
- Schuch A., Schuchmann H., Gaukel V. (2015) Rheology of Emulsions. In: Drioli E., Giorno L. (eds) Encyclopedia of Membranes. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-40872-4_1884-1.
- Moreton R.C. (2010) Commonly Used Excipients in Pharmaceutical Suspensions. In: Kulshreshtha A., Singh O., Wall G. (eds) Pharmaceutical Suspensions. Springer, New York. https://doi.org/10.1007/978-1-4419-1087-5_3.
Pharmaceutical Thickeners (Viscosity-Increasing Agents): Overview, Types, and Functions
Thickening agents are usually high molecular weight polymer excipients commonly used to increase viscosity of formulations. This can serve several objectives, including stability, ease of use and drug delivery. In this post, we provide an overview of materials frequently used to thicken pharmaceutical products.
Definition of Pharmaceutical Thickeners
Pharmaceutical thickening agents (thickeners, for short) are a diverse group of excipient materials used to increase the viscosity of liquid pharmaceutical systems, ideally without altering other fundamental properties.
They are also commonly known as viscosity-increasing agents.
Viscosity is the internal friction that occurs between the molecules within a liquid. It is a measure of the resistance to deformation in shear, such as flow or pouring, and is due to intermolecular friction and molecular adhesion-cohesion within the fluid.
Viscosity of a fluid decreases with increase in temperature due to increased atomic/molecular mobility, which decreases intermolecular cohesion and friction. The presence in solution of a high concentration of solutes can have an appreciable impact on viscosity of the solution.
This is why the terms “thick” (concentrated and high viscosity) and thin (dilute and low viscosity) are used as qualitative descriptions of the viscosity of liquid systems.
Many thickeners form weakly cohesive colloidal structures, which can exist as solutions, suspensions or gels when dissolved or dispersed in a suitable liquid medium.
Note that thickening agents are sometimes (and mistakenly) referred to as suspending agents. Although there is overlap in the type of excipients used for both product categories, there are sufficient differences to warrant a clear separation of the two categories, an approach we have taken in this article.
How Thickeners Increase Solution Viscosity
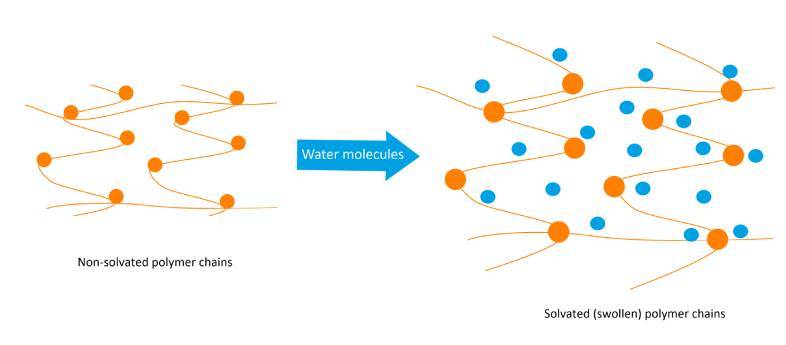
With respect to the use of polymers, the addition of a high molecular weight polymer increases viscosity of the liquid in which it is dissolved. The increase in viscosity is caused by strong internal friction between the randomly coiled and/or swollen macromolecules, and the surrounding solvent molecules, through hydrogen bonding¹. This is illustrated below:
How Thickeners Increase Solution Viscosity
With respect to the use of polymers, the addition of a high molecular weight polymer increases viscosity of the liquid in which it is dissolved. The increase in viscosity is caused by strong internal friction between the randomly coiled and/or swollen macromolecules, and the surrounding solvent molecules, through hydrogen bonding¹. This is illustrated below:
Functions of Pharmaceutical Thickeners
Viscosity is one of the most important properties associated with liquid systems, and formulations that possess high viscosity have many practical applications within the pharmaceutical space, as outlined below:
Drug delivery
The rate of drug delivery is influenced by viscosity of the milieu. Liquid and semisolid topical and oral formulations, as well as parenteral and matrix-based solid dosage forms frequently utilise high viscosity systems to slow down rates of drug diffusion.
Demulcent properties (soothing, irritation reduction & increase in slip)
Demulcents are a class of materials used to lubricate and protect mucous membranes, and more especially the oral, pharyngeal, oesophageal, and gastric mucosa. Their action depends on having sufficient tack and slip, both of which are obtained when polymeric excipients are dissolved in aqueous media. Click here to read about the challenges of dysphagia and oral medicines, and what action is required if we are to address it.
Substantivity and film formation
The ability of a formulation to adhere and remain in place (contact time) is a function of, among other factors, viscosity and film formation. Viscosity-increasing agents are routinely used to increase substantivity and contact time of topical and bioadhesive formulations.
Suspending and stability enhancement
Thickeners are typically used to minimise separation and settling that typically follow when insoluble ingredients are suspended in liquids
Improvement of appearance, consistency and quality
Thickening agents are added to skincare formulations to give skincare products a more appealing consistency and smoothness. Consistency is a particular formulation characteristic that describes the firmness or runniness of a product.
Gelling and texturizing
A key property of hydrocolloids (such as pectin and xanthan gum) is their ability to form gels and thicken liquid product. For instance, pectin, carrageenan and agar form gels under specific conditions, which opens up a myriad of applications and functionalities.
Like what you're reading? Share it with your network.
Types of Pharmaceutical Thickening Agents
A wide range of excipients can be used as thickening agents, with the most common being hydrocolloid gums and cellulose ethers.
Hydrocolloid Gums
The term ‘hydrocolloid’ is derived from two Greek words, ‘hydro = water, and ‘kolla’ = glue. Currently, the term hydrocolloid is used for macromolecular (polymers) hydrophilic substances (with affinity for water), and includes polysaccharides and proteins.
When dissolved or dispersed in water, hydrocolloids hydrate and swell (increase in volume) due to solvent molecules penetrating into void spaces that exist between polymer chains. Hydration is accompanied by an increase in viscosity.
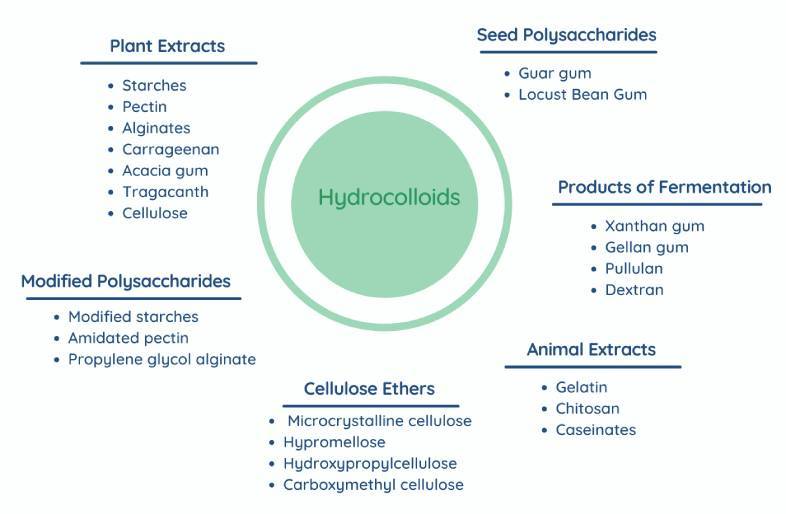
Hydrocolloids can be classified into four groups:
Plant-derived hydrocolloids: including pectin, agar, Alginates, acacia, tragacanth, Karaya gum, guar gum, starches, cellulose and locust bean.
Products of fermentation: including xanthan gum, dextran, gellan gum and pullulan.
Semi-synthetic hydrocolloids: including modified starches, cellulose ethers (Methylcellulose, Hypromellose, Ethylcellulose, Hydroxypropylcellulose and hydroxyethylcellulose), amidated pectin and propylene glycol alginate.
Animal derived hydrocolloids: such as gelatin, chitosan and caseinates.
Vinyl polymers
Vinyl polymers is a generic term given to high molecular weight synthetic polymers based on the vinyl groups: XCH=CHY. In the pharmaceutical field, the most important vinyl polymers are:
Water soluble polyvinylpyrrolidones: This group includes povidone and copovidone of molecular weights ranging from 2500 to 1250000 g/mol.
Ethylene-vinyl acetates: Ethylene vinyl acetates (EVAs) are copolymers of ethylene and vinyl acetate monomers produced by free radical polymerisation under high temperature and pressure.
Varying vinyl acetate composition impacts polymer properties such as melting point, crystallinity and polarity, solubility, transparency, hardness and compatibility with active ingredients.
EVAs are mainly applied in the formulation of transdermal drug devices and Class I – IV medical devices, where they are used as and/or incorporated into matrix formers, rate controlling membranes and backing layers. EVAs provide tackiness, bioadhesion and flexibility.
Polyvinyl alcohol: Polyvinyl alcohol (PVA) is a water-soluble and biodegradable polymer obtained by polymerisation of vinyl acetate followed by partial hydrolysis. Pharmaceutical grades typically exhibit a degree of hydrolysis of 88% and molecular weight that vary from 20 000 g/ml to over 100 000 g/mol.
Clays (Aluminium Silicates)
Clays are fine-grained naturally-occurring aluminium silicates with traces of other inorganic oxides. They typically consist of plate-like crystal habits. The most well-known clays in the pharmaceutical field are bentonite, hectorite and magnesium aluminium silicate.
Owing to their platy habits, aluminium silicate particle planes possess different surface charge characteristics and complex modes of particle-particle interaction, depending on concentration and pH, among other factors.
In suspension, hydrodynamic forces or the influence of electroviscous effects of clay solutions impacts rheology and viscosity of clay suspensions, much the same way as polymers dispersions.
Carbomers
Carbomer are a group of closely related synthetic, high molecular weight, nonlinear polymers of polyacrylic acids, cross-linked with a polyalkenyl polyether. They differ in molecular weight, polymerization solvent or side-groups and contain between 56 and 68% w/w carboxylic acid groups.
Carbomer are highly versatile and multifunctional excipients for oral (solid and liquids) and topical formulations. When hydrated and neutralised, they yield highly viscous gels with viscosities upwards of 40,000 mPa s for concentrations as low as 0.5% w/v.
If you’re interested in learning more about carbomers, we have prepared a quick reference guide which you access through this link:
Carbomers: Overview, key properties and formulating tips
Polyols, sugar and oligosaccharide
Concentrated solutions of sugars, oligosaccharides and polyols behave very much like high molecular weight polymer solutions owing to increased intermolecular attractions between sugar molecules (hydrogen bonds) and the solvent. When sheared, the interactions create a drag which manifests as an increase in viscosity.
This class of viscosity-increasing agents include liquid Sorbitol, liquid Maltitol, sucrose, fructose, dextrose, maltodextrin and Polydextrose.
Miscellaneous Thickening Agents
Polyethylene glycol and polyethylene oxide: Polyethylene glycol and polyethylene oxide are related non-ionic polymers formed by the reaction of ethylene oxide and water under pressure in the presence of a catalyst. Low molecular weight polyethylene glycol grades are liquids and used primarily as solvents and co-solvents in oral and topical formulations. Solid polyethylene glycol grades are used suspending agents or to adjust the viscosity and consistency of other suspending vehicles.
Silica: Also known as colloidal silicon dioxide, silica is an oxide of silicon with the chemical formula SiO2. Silica grades with a high specific surface area are used to thicken polar liquids thereby converting them into transparent gels. This effect can be used as stabilising strategy for emulsions and semisolid preparations.
Other materials which can be used for thickening formulations include:
- Silicones
- Glycerine
- Fatty acids
- Hard fats
Summary of Thickening Agents
Many pharmaceutical excipients are often added to formulations to increase the viscosity or thicken liquid formulations. This property can be utilised to improve product properties, including drug delivery, stabilisation, demulcent effects, and texturizing. The range of materials that serve this function is very broad, but typically includes hydrocolloid gums, synthetic polymers, sugars and polyol syrups and many other miscellaneous materials.
Sources and Cited References
Pharmaceutical Diluents and Fillers: Overview, Types, and Uses
Diluents and fillers are a ubiquitous class of materials in formulated products, including pharmaceuticals, medical devices, vaccines, nutraceuticals, cosmetics and industrial goods. But what exactly are diluents and fillers? Do they perform any function beyond filling up space? This technical note provides an introductory review of pharmaceutical diluents and fillers. The information will be particularly relevant to anyone interested in learning more about fillers and diluents.
Definition of Pharmaceutical Diluents and Fillers
For the vast majority of medical products in current use, the active pharmaceutical ingredient is rarely administered in its basic form. Often, this is because it is present in much lower amounts relative to the weight of the dosage form unit.
A specific type of excipients known as bulking agents are therefore added to product formulations to provide bulk and render the product convenient to process, manufacture or administer.
Alternative names for fillers and diluents are:
- carriers
- fillers
- diluents
- extenders
- voluminising agents
Not every formulation requires a diluent – in product formulations where the active ingredient or other functional excipients are already added in sufficient proportions, for instance, greater than 70 – 90% a bulking agent may not be required.
When added, however, and despite their humble name, pharmaceutical fillers do a lot more than just fill space. Here are some of their functions:
- add structure
- aid application e.g., mouthfeel
- impact appearance
- influence many quantifiable properties that are important to a product’s performance.
In solid dosage forms, fillers and diluents are almost always required as they provide the foundation upon which the formulation is constructed.
For convenience of administration (holding and swallowing, for instance), a tablet should weigh no less 50mg, and where the active ingredient is present in very low quantities, a diluent with high carrying capacity is absolutely essential. This is an important consideration, particularly today given the increasingly high percentage (and growing) demographic of seniors across the world. Click here to read about the importance of applying empathy in new drug development.
As shown in the graphic below, diluents are often present in the highest concentration.
Diluents and fillers are not limited solid dosage forms only – their usage also applies to many other product types, including topical creams and lotions, parenteral products, dusting powders, aerosols, suppositories, and suspensions.
Types of Pharmaceutical Diluents and Fillers
Pharmaceutical fillers are very diverse group of excipients. They may be:
- organic chemicals
- mineral substances
- metal oxides
- polymers
- simple inorganic chemicals,
- liquids,
- gases (aerosols)
They may be classified on the basis of
- source (natural, mineral or synthetic)
- dosage form in which they are used
- physical and chemical properties and
- functionality
A more practical approach is to classify fillers as either functional or non-functional.
Functional fillers are those excipients that extend the property gamut of the formulation, opening up new performance qualities and functionalities. For example, silica is frequently added to elastomers and rubbers to provide to the topical medical device strength and structure without compromising drug delivery properties.
Non-functional fillers, as the name suggests, only serve one thing – to provide bulk and fill void volume in the formulation, which is still an important requirement in the formulation for the reasons outlined previously.
The image galery below shows fillers that may be encountered in the pharmaceutical industry:
- maltodextrin (used as a carrier for a pharmaceutical flavour)
- native maize starch
- compressed chewing gum base
Ideal Properties of a Pharmaceutical Filler and Diluent
When it comes to pharmaceutical formulations, there are a number of fundamentals properties that formulators seek in fillers:
- Physiological inertness
- Filler concentration
- Particle size and size distribution of the filler
- Shape and aspect ratio
- Bulk density/carrying capacity
- Low API binding capacity
- Processability
- Physical and chemical stability
- Strength (low impact)
- Cost-effectiveness
For solid dosage forms (tablets and capsules), the following factors (in no particular order) are the most important considerations when selecting a material to use:
- Compressibility and compactibility
- Flowability
- Particle size and distribution
- Moisture content and type of interactions
- Bulk density
- Compatibility
- Solubility and effect on bioavailability
- Abrasiveness (lubricity)
- Stability
- Physiological inertness
- Cost and availability
- Regulatory acceptance
The 10 Most Popular Filler-Diluents in Solid Dosage Forms
The following fillers are consistently the most commonly used materials in solid dosage forms:
Lactose
Lactose is a natural disaccharide obtained from milk. It consists of one galactose and one glucose moiety. It is listed in the USP-NF, JP and PhEur and is also GRAS listed and included in the FDA Inactive Ingredients Database (IM, and SC: powder for injections; oral: capsules and tablets: inhalation preparations; vaginal preparations).
Lactose is one of the most widely used filler and diluent in tablets and capsules, and to a more limited extent in lyophilized products and infant formulas. Lactose is also used as a diluent in dry-powder inhalation.
Find more about lactose through these links on our site:
Microcrystalline cellulose
Microcrystalline cellulose is purified, partially depolymerized cellulose and one of the most widely used ingredients in the pharmaceutical industry. It is used in many types of dosage forms, including as filler, binder, flow aid, lubricant, texturiser, among others.
Microcrystalline cellulose is listed in all major pharmacopoeia, including the USP-NF, Ph.Eur, BP, JP and IP. It is also GRAS listed and accepted for use as a food additive in Europe and the United States and included in the FDA Inactive Ingredients Database (oral: powders, suspensions, syrups and tablets; topical & vaginal products).
Find more about the uses of microcrystalline cellulose through these links on our site:
- Microcrystalline cellulose
- 101 interesting facts about microcrystalline cellulose
Starch
Starch is a polysaccharide consisting of two polymers: a linear polymer known as amylose and a branched polymer known as amylopectin. Both polymers are made up of glucose residues.
Pharmacopoeia have individual monographs for different starch derivatives, including native starch, pregelatinised starch, tapioca starch, pea starch and rice starch. Starch is also co-processed with lactose and microcrystalline cellulose.
Generally, as a material, starch is a highly versatile material, finding application in many different dosage forms. It is widely used as filler and diluents in solid dosage forms. It is also added to dusting powder and also in topical products and film coatings.
Find more about the uses of starch through these links:
- Substituting lactose for starch in solid dosage forms
- Benefits of using high performance excipients
Calcium phosphate
Calcium phosphate is actually a family of chemical substances made up of calcium ions and phosphate anions. These include anhydrous dibasic calcium phosphate, hydrated dibasic calcium phosphate, and tribasic calcium phosphate. All the three grades are listed in the main pharmacopoeia, are GRAS listed and included in the FDA Inactive Ingredients Database.
The main advantages of calcium phosphates as filler-diluents include:
- Chemical inertness
- Low hygroscopicity
- High density
- Source of calcium
- Natural source
Find out more about uses of calcium phosphates through the following links:
Calcium carbonate
Calcium carbonate is the calcium salt of carbonic acid. It is listed in all the major pharmacopoeia and is GRAS listed. The FDA Inactive Ingredients Database lists calcium carbonate as an excipient for chewing gum, capsules and tablets.
It is one of the main fillers used in compressed tablets. Other uses are as a bulking agent in tablet sugar-coating processes and as an extender and opacifier in tablet film-coatings.
Sucrose
Sucrose is a naturally-occurring disaccharide made up of a glucose molecule joined to a fructose molecule. Pharmaceutical grade sucrose is currently obtained from sugar cane and sugar beet, and is listed in all the major pharmacopoeia and the FDA Inactive Ingredients Database (parenterals, tablets, capsules, syrups and topical products). It is a highly compressible filler in tablets and selected for its sweetness, low reactivity, safety profile and cost-effectiveness.
Maltodextrin
Maltodextrin is a saccharide consisting of mixture of polymers of D-glucose units. It is prepared by the partial hydrolysis of a starch. It is a non-sweet, odourless, white powder or granules.
Maltodextrin is used as a coating agent, tablet and capsule diluent, tablet binder and viscosity- increasing agent. Maltodextrin is also widely used in confectionery and food products, as well as personal care applications.
Mannitol
Mannitol (D-mannitol) is a polyol and an isomer of sorbitol. It occurs as a white, crystalline powder, or free-flowing granules, with a sweet taste. It is noted for its cooling, sweet sensation in the mouth. It is official in multiple pharmacopoeia as a filler diluent as well as a therapeutic agent.
Mannitol is used as a diluent (10 – 90% w/w) in tablet formulations. It’s specific advantage is its low hygroscopicity and high compressibility. However, it is a costly filler compared with lactose and selected for use where mouthfeel is important, for example, chewable tablets.
Sorbitol
Sorbitol is a polyol and an isomer of mannitol. It is supplied as a white or almost colourless, crystalline, hygroscopic powder. It is available in a wide range of grades and polymorphic forms, such as granules, powders and pellets for use in solid and liquid oral and topical products.
Sorbitol is used as a diluent in tablet formulations prepared by either wet granulation or direct compression. It is especially useful in chewable tablets owing to its pleasant, sweet taste and cooling sensation.
Sodium Chloride
Sodium chloride is an inorganic chloride salt having sodium as the exchangeable ion. It is listed in all the major pharmacopoeia, is GRAS and included in the FDA Inactive Ingredients Database (injections, inhalations, nasal, ophthalmic, oral, otic, rectal and topical products). It occurs as a white, crystalline powder with a saline taste.
Sodium chloride is used widely in parenteral products to produce isotonic solutions. In oral products, it functions as a diluent (capsules and direct compression tablets), and a porosity modifier in controlled-release coatings.
| Material | Reason for Popularity | Usage |
|---|---|---|
| Lactose (all grades) | Highly compactible | Up to 60% w/w in tablets & capsules |
| Microcrystalline cellulose | Versatile, inert and low cost | Optimum level is 15-20% w/w in tablets & capsules |
| Starch (all grades) | Versatile, inert and low cost | Up to 40% w/w as a filler in tablets & capsules |
| Calcium phosphates | Low moisture content. Source of Calcium | Up to 60% w/w in tablets & capsules |
| Sucrose | Highly compactible & Sweet tasting | Highly compressible grades up to 90% w/w in tablets, capsules & oral liquids |
| Maltodextrin | Highly compactible | Up to 50% w/w/ in tablets & capsules |
| Mannitol | Mouthfeel & highly compactible and inert | Up to 60% w/w in tablets & capsules |
| Sorbitol | Mouthfeel & highly compactible and inert | Up to 60% w/w in tablets, capsules & oral liquids |
Summary about pharmaceutical Fillers and Diluents
Despite their ordinary name, pharmaceutical fillers do much more than simply fill up space. They provide structure, influence appearance, and impact many other measurable properties that ultimately determine product’s end uses.
For solid dosage forms, lactose and microcrystalline cellulose are the most commonly used diluents. Mannitol is used as a substitute for lactose, however it is costly and its use is only justified when other functional properties are sought in a formulation.
Sources Used
Pharmacentral has a strict referencing policy and only uses peer-reviewed studies and reputable academic sources. We avoid use of personal anecdotes and opinions to ensure the content we present is accurate and reliable.
- A. Crouter, L. Briens, The effect of moisture on the flowability of pharmaceutical excipients, AAPS PharmSciTech, 15 (2014) 65-74. Pubmed
- A. Lura, G. Tardy, P. Kleinebudde, J. Breitkreutz, Tableting of mini-tablets in comparison with conventionally sized tablets: A comparison of tableting properties and tablet dimensions, International journal of pharmaceutics: X, 2 (2020) 100061. Pubmed
- N.O. Sierra-Vega, K.M. Karry, R.J. Romañach, R. Méndez, Monitoring of high-load dose formulations based on co-processed and non co-processed excipients, Int J Pharm, 606 (2021) 120910. Pubmed