What is Gellan Gum?
Gellan gum is a water soluble anionic hydrocolloid produced by the microorganism Sphingomonas elodea. This microorganism was discovered in 1978 in the United States by scientists at Merck following a concerted effort to find naturally occurring hydrocolloids.
Gellan gum is supplied as a free-flowing white powder. For commercial grades, gellan gum is manufactured by fermentation of a carbohydrate. In its native state, Gellan gum has acyl groups in its structure. Treatment with alkali removes acyl groups completely
Physicochemical Properties of Gellan Gum
Chemical Structure
Gellan gum is a straight chain polymer consisting of D-glucose, L-rhamnose and D-glucuronic acid units. In its native or high acyl grade, acetate and glycerate substituents are present on one of the glucose residues. The low acyl grade there is no acyl substituents. Note that the presence of acyl groups has a strong bearing on gel properties of Gellan gum.
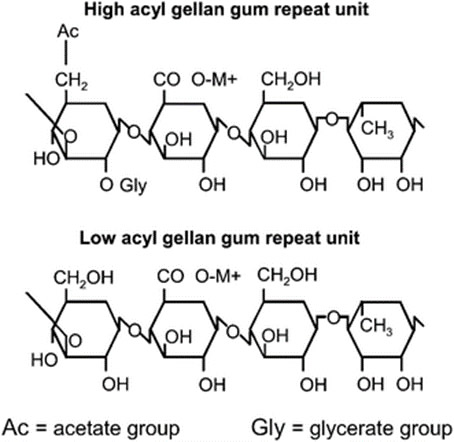
Differences between High Acyl and Low Acyl Gellan Gum
| High Acyl Gellan Gum (KELCOGEL® LT100 | Low Acyl Gellan Gum | |
| Molecular weight | 1 – 2 x106 Daltons | 2 – 3 x105 Daltons |
| Solubility | Hot water | Cold or hot water |
| Set Temperature (oC) | 70 – 80 | 30 – 50 |
| Thermoreversibility | Thermoreversible | Heat stable |
Where can you use Gellan gum?
Gellan gum is a useful and effective gelling agent in pharmaceutical and food products. It offers the following benefits:
- It is effective at low concentrations
- Provides a wide range of viscosities and textures
- Gels on cooling
- Forms fluid gels, which are solutions with a weak gel structure. These systems are extremely versatile for suspending drug substances without settling
- Can be used in combination with other hydrocolloids
Uses of Gellan gum in pharmaceutical products
| Application | Typical products |
| Oral suspensions (immediate and sustained release) | Ibuprofen, Paracetamol, Cetirizine |
| In-situ forming gels | Nasal and ophthalmic products |
| Medicated gummies | Vitamins and children medicines |
| Hair care products | Stabilization of medicated shampoo formulations |
| Topical products | Creams and lotions as a substitute for paraffins |
| Tablet coatings | To improve slip and enhance swallowing |
| Oral care | In toothpaste formulations to bind actives while creating a gel-like texture |
Regulatory status
Approved for use in foods in Europe, USA, Japan, China and India. Gellan gum is also approved for use in non-food, cosmetics and pharmaceutical formulations in the USA, Canada, Australia, Brazil and China. Pharmaceutical use in EU falls under E418 (Directive EC/95/2). Gellan gum is manufactured in accordance with applicable food GMPs and complies with purity criteria defined in the current USP-NF monograph.
References
KELCOGEL® Gellan gum book, 5th Edition, CP Kelco, San Diego, USA
Mahdi M H et al., 2014. Evaluation of Gellan gum fluid gels as modified reléase oral liquids. International Journal of Pharmaceutics, 475; 335 – 343.
Kubo W et al., 2003. Oral sustained delivery of paracetamol from in-situ gelling Gellan and sodium alginate formulations. International Journal of Pharmaceutics, 258 (1-2); 335 – 343; 55-64

