Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Tribasic Calcium Phosphate (also known as Tricalcium phosphate or TCP) is an inorganic mineral excipient and calcium salt of orthophosphoric acid. It corresponds to the chemical formula Ca3(PO4)2 and the Ca/P ratio is 1.5. Although it occurs naturally in the mineral apatite, it is obtained synthetically by reacting calcium hydroxide with orthophosphoric acid. Tribasic calcium phosphate is supplied as a white, odourless, tasteless powder or crystalline solid.
Synonyms and Trade Names: Calcium Orthophosphate; Hydroxylapatite; Precipitated Calcium Phosphate; Tertiary Calcium Phosphate; Tricalcium Diorthophosphate; E341; TRI-CAL WG; TRI-TAB; Tri-Cafos
Pharmacopoeial Compliance: USP-NF; Ph.Eur; IP; J.P; FCC
Uses and Applications: Anticaking Agent; Buffering Agent; Dietary Supplement; Glidant, and Tablet and Capsule Diluent

Tribasic calcium phosphate is an inorganic compound and a calcium salt of phosphoric acid. It is also known as Tricalcium phosphate, TCP, Tertiary calcium phosphate, and bone phosphate of lime (BPL). It is described in the Ph.Eur as a mixture of Calcium phosphates, with the amount of Calcium ranging between 35.0% and 40.0%. In the USP-NF, Tribasic calcium phosphate is specified as consisting of variable mixtures of Calcium phosphates, with an approximate composition of 10CaO.3P2O5.H2O.
As implied in the compendial definitions, Tribasic calcium phosphate is not a single chemical entity, and the term is used to refer to multiple calcium phosphates, having several chemical names, crystal habits, and molecular formulas, depending on the preparation method and/or source. For this reason, there are multiple chemical identifiers (CAS registry numbers) assigned to different substances which all bear the name, Tribasic calcium phosphate.
Chemically synthesised Tribasic calcium phosphate is obtained by precipitation of Calcium hydroxide and orthophosphoric acid and a calcinating programme at >1000 oC. The material obtained through this route can be represented by the chemical formula Ca3(PO4)2 and stoichiometrically possesses a Ca/P ratio of 1.5. It occurs in multiple polymorphs, including α, β, γ and super α-although only β-TCP is stable enough and used in medical and pharmaceutical fields.
Tribasic calcium phosphate also occurs naturally in the mineral rock deposits (such as apatite and whitlockite). This grade of TCP is sometimes known as Calcium hydroxide phosphate, Hydroxyapatite or Hydroxylapatite (HA), and corresponds to the chemical formula Ca5(PO4)3(OH), although it is usually written Ca10(PO4)6(OH)2 to signify that the crystal unit comprises two entities. Up to 50% by volume and 70% by weight of human bone is a form of hydroxyapatite. Furthermore, a great proportion of naturally-sourced commercial Tribasic calcium phosphate is powdered Hydroxyapatite.
Tribasic calcium phosphate is a white, odourless and tasteless powder.
Tribasic Calcium Phosphate
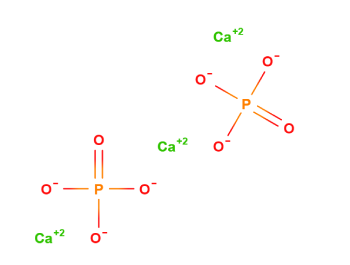
Calcium Hydroxyphosphate
| Chemical Name | Calcium phosphate is not a clearly defined chemical entity but is a mixture of calcium phosphates, with several chemical names, CAS Registry Numbers, and molecular formulas. The most frequently cited are shown are Calcium hydroxide phosphate and Tricalcium orthophosphate. |
| CAS Registry Number
Calcium hydroxide phosphate Calcium phosphate |
12167-74-71 7758-87-4 |
| Empirical Formula and Molecular Weight
[Ca5(OH)(PO4)]x Ca3(P04)2 |
310.20
502.32 |
| EC Number | 231-840-8 |
| FDA UNII Code | K4C08XP666 |
Tribasic calcium phosphate is an approved pharmaceutical excipient. It is listed in all the major pharmacopoeia, including the USP-NF, Ph.Eur, and J.P. It is GRAS listed and included in the US FDA Inactive Ingredients Database. Tribasic calcium phosphate is also accepted for use as a food additive in Europe (E341 iii). A specification for Tribasic calcium phosphate tribasic is contained in the Food Chemicals Codex (FCC).
| Form | Solid, powder |
| Appearance | White powder |
| pH (20% slurry) | 6.8 |
| Bulk density | 0.3 – 0.8g/ml depending on grade |
| Tapped density | 0.95 g/ml (granular grades) |
| True Density | 3.14 g/ml |
| Flowability | 25.0g/s for granular grade |
| Melting point | 1670 0C |
| Moisture content | Slightly hygroscopic. At RH values between about 15% and 65%, the equilibrium moisture content at 25 oC is about 2.0% |
| Particle size distribution | Dependent on grade and supplier.
Fine grades – 5-10µm; 99% of particles < 44µm. Coarse grades – 80-600 µm; 99% of particles <420µm |
| Solubility | Soluble in dilute mineral acids; very slightly soluble in water; practically insoluble in alcohols |
| Specific surface area | 70 – 80 m2/g |
| Test | PhEur 6.4 | USP32-NF27 |
| Official name | Calcium Phosphate | Tribasic Calcium Phosphate |
| Authorised use | specified | specified |
| Definition | specified | specified |
| Identification | specified | specified |
| Characters | specified | n/a |
| Loss on ignition | ≤ 8.0% | ≤ 8.0% |
| Water-soluble substances | n/a | ≤ 0.5% |
| Acid-insoluble substances | ≤ 0.2% | ≤ 0.2% |
| Carbonate | n/a | specified |
| Chloride | ≤ 0.15% | ≤ 0.14% |
| Fluoride | ≤ 75 ppm | ≤ 0.0075% |
| Nitrate | n/a | specified |
| Sulfate | ≤ 0.5% | ≤ 0.8% |
| Arsenic | ≤ 4 ppm | ≤ 3 ppm |
| Barium | n/a | specified |
| Iron | ≤ 400 ppm | n/a |
| Dibasic salt and calcium oxide | n/a | specified |
| Heavy metals | ≤ 30 ppm | ≤ 0.003% |
| Assay (as Ca) | 35.0 -40.0% | 34.0 -40.0% |
| Labelling | n/a | specified |
Key: n/a Specification is not listed
*All claims with respect to conformity are subject to our Terms and Conditions. No express or implied warranty is made for specific properties or fitness for any particular application or purpose.
Tribasic calcium phosphate is a pharmaceutical excipient commonly used as an anticaking agent, buffering agent, glidant, tablet and capsule diluent-filler, and also as a dietary supplement. The most common application is as a tablet and capsule filler/binder in either direct-compression or wet-granulation processes.
In dietary supplements, Tribasic calcium phosphate functions both as a filler, binder and a source of both calcium and phosphorus, the two main estrogenic minerals for hone health. It provides a higher calcium load than dibasic calcium phosphate and a higher Ca/P ratio. Generally, one gram of tribasic calcium phosphate represents approximately 10.9 mmol of calcium and 6.4mmol of phosphate. Since the bioavailability of the calcium is improved by the presence of cholecalciferol, Tribasic calcium phosphate and Vitamin 3 can be combined in the same formulation as a cost-effective solution to prevent bone fracture prevention in vulnerable.
In dry powdered products (sachets) Tribasic calcium phosphate powder can be used as an anticaking agent and an alternative to hydrophobic silica. It is similarly used in food products as an anticaking agent.
As an excipient, Tribasic calcium phosphate is generally regarded as non-toxic and non-irritant at the levels employed as pharmaceutical formulations. However, ingestion or inhalation of excessive quantities may result in the deposition of Tribasic calcium phosphate crystals in the body, potentially causing inflammation and lesions where it is deposited. Reportedly, consumption of large amounts is associated with abdominal distress, including nausea and vomiting.
Toxicology: LD50 (rat, oral): > 1 g/kg
Tribasic calcium phosphate is a chemically stable material, and is also not liable to cake formation during storage. However, the bulk material should be stored in a well-closed container in a cool, dry place. When handling Calcium phosphate, observe SHEQ protocols appropriate to the circumstances and quantity of material handled. Eye protection and gloves are recommended. The material should be handled in a well-ventilated environment since dust inhalation may be an irritant. The use of a respirator is recommended.
Tribasic calcium phosphate occurs in natural mineral and rocks (hydroxylapatite, voelicherite, and whitlockite) although it may be obtained obtained synthetically using widely available non-critical resources. Being inert and non-toxic, it is considered safe for the environment, with minimal long-term impact on ecology or marine life. Tribasic calcium phosphate achieved a total score of 77/100 by the Excipients Forum Sustainable Chemistry™ assessment scheme.
Tung M.S. (1998) Calcium Phosphates: Structure, Composition, Solubility, and Stability. In: Amjad Z. (eds) Calcium Phosphates in Biological and Industrial Systems. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-5517-9_1
[2] P.C. Schmidt, R. Herzog, Calcium phosphates in pharmaceutical tableting. 2. Comparison of tableting properties, Pharmacy World & Science : PWS, 15 (1993) 116-122.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.