Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
Hydrated Colloidal Silica (also known as Precipitated Silicon Dioxide) is a synthetic amorphous silicon dioxide obtained by precipitation, hence the alternative name, Precipitated silica. It is available in different grades, which are produced by modifying the specific surface area and particle size during production. Hydrated colloidal silicon dioxide is supplied as a white or almost white, light, fluffy and extremely fine powder.
Synonyms and Trade Names: Precipitated Silicon Dioxide; Synthetic Silica; Synthetic Amorphous Silica; Hydrated Colloidal Silica; Precipitated Silica; Hydrated Silica; Colloidal Silicon Dioxide Hydrated; Synthetic Hydrated Silicon Dioxide; E551; SIPERNAT®; SIDENT®; SYLOID ®; SPHERILEX®
Pharmacopoeial Compliance: USP-NF; Ph.Eur; JPE
Uses and Applications: Absorbent for Oily Formulations; Carrier, Rheology Modifier, Anticaking Agent; Clarifying Agent; and Grinding Aid

Hydrated colloidal silica is a form of synthetic amorphous silicon dioxide obtained by precipitation (hence the name, “precipitated “silica”). The following reactions are involved in the production process:
Being a product of silicon dioxide, Hydrated colloidal silica shares the chemical formula, SiO2 with other synthetic amorphous silica products, but has a different CAS Registry number of [112926-00-8]. It is distinguishable from Anhydrous colloidal silica (which is prepared by the flame hydrolysis of chlorosilanes) in two key respects: (1) it contains variable amounts of water in its formula, hence the the alternative name, Silicic anhydride, and (2) it has a higher specific surface area due to the presence of porous particle structures, giving a higher propensity to absorb liquids.
In the production process, the sodium silicate (waterglass) undergoes controlled neutralisation with either concentrated sulphuric acid or hydrochloric acid. The process is undertaken in an aqueous medium at temperatures of >60 °C. The Silica precipitates out as a slurry of hydrated silica, which is washed and filtered to remove by-products. It is then dried in hot air and milled or passed through a classifier. The method and conditions set up during production produce a type of synthetic silica that is structurally and characteristically different, including exhibiting internal pore volume.
Hydrated colloidal silicon dioxide is supplied as a white or almost white, light, fluffy and fine powder. It is commercially available in multiple grades, which are produced by modifying the specific surface area and particle size.
Silicon and oxygen are the two most abundant elements in the earth’s crust. In nature, silicon almost always exists in combination with oxygen, either as free silica (SiO2), in conjunction with other elements (for instance, in silicates, which are the main minerals in rocks and soil), or as combined silica (SiO3). The different silicon compounds have substantially different chemical properties, applications, and hazards.
Free silica (Silicon dioxide) is a hard, low-reactivity, colourless substance that occurs naturally in rocks and minerals or can also be industrially produced in the form of synthetic amorphous silica. All forms of Silica, whether natural, synthetic, crystalline, cryptocrystalline or amorphous, are assigned a single CAS Registry number [7631-86-9]. For convenience, the different forms of Silica can be divided into three main groups as shown in the chart below:
At the fundamental level of Silica’s chemical properties is the Silica tetrahedron (SiO4), which consists of a central Silicon cation covalently bonded to four oxygen atoms, arranged in the shape of a tetrahedron. Silica tetrahedra may be linked and arranged in a variety of ways, from simple to complex three-dimensional frameworks. Crystalline forms of Silica exhibit a highly ordered crystal lattice, determined by the ordered arrangement of the Silica tetrahedra. Amorphous forms, on the other hand, have random, disordered lattices. The orientation of the bonds is random, and there is no long-range periodicity.
To differentiate between the different silica analogues, new CAS Registry numbers have been assigned. These are shown in the chart below:
![]()
Synthetic amorphous silicon dioxides are further divided into three main types, namely:
Even though they share the same chemical structure and synthetic origin, Synthetic silicas exhibit different properties, as briefly reviewed below:
Pyrogenic silicon dioxide is produced using a high-temperature process in which silicon tetrachloride is vaporised in an oxygen-hydrogen atmosphere according to the following chemical reaction:
SiCl4 + 2H2 + O2 -> SiO2 + 4HCl
The raw materials used in the silica production process are all inorganic and very pure. As a result, the synthesis produces only hydrochloric acid (which is easily removed) and silica in very high states of purity (typically > 99.9%). Silicon dioxide produced pyrogenically exists in the form of chain-like, branched aggregates, giving rise to a fluffy, light powder. (The term “fume” alludes to the method of manufacture, which involves the use of a flame.) Varying processing conditions allows the production of silica products with different specific surface areas, typically between 50 and 400 m2/g.
The pyrogenic method for producing Silica was invented in 1941 by Harry Klopfer, a scientist at Degussa (now part of Evonik AG). This method is what is still used by Evonik (for the production of pyrogenic silica marketed under the AEROSIL® brand name) and Cabot Corporation (for the Cab-O-sil® fumed silica brand). Note that these silica grades can be used in their native (unprocessed) state. They can also be further processed (for example, spray drying, granulation, or surface chemical modification) to turn them into other technical silica grades.
Precipitated silicon dioxide is silica produced in an aqueous solution at temperatures >60 °C. In this process, sodium silicate (waterglass) undergoes controlled neutralisation with either concentrated sulphuric acid or hydrochloric acid. The Silica precipitates out as a slurry of (hydrated) silica, which is washed and filtered to remove by-products. It is then dried in hot air and milled or passed through a classifier.
Precipitated silicon dioxide has been known since the mid 17th century. It was not until the 1920s that its practical uses and industrial production were fully established. Currently, Precipitated silica is produced in volumes that are up to x10 greater than for Pyrogen silica. The method and conditions have been fine-tuned and now permit production of many types of synthetic silica that are structurally and characteristically different, including exhibiting internal pore volume/specific surface area, larger particle sizes, and water content.
3). Surface-Modified Silicon Dioxide
The Silica grades described thus far are available for use as in their native or unmodified state. These materials have freely accessible silanol groups (Si-OH) on the surfaces of Silica particles, rendering them hydrophilic. Frequently, it is desirable to have hydrophobic silica, i.e a product that repels water. Hydrophobicity can be achieved through a post-synthesis step in which the silanol groups are reacted with organic groups. The added organic groups are tightly bound to the surface (via covalent bonds) and are only broken via thermal decomposition.

Pharmaceutical-approved hydrophobic silica is produced by reacting hydrophilic silicon dioxide with dimethylchlorosilane immediately after the production of Silica particles in the hydrogen flame chamber. This process is also conducted at high temperatures and allows dimethysilyl groups to be bound irreversibly onto the surface of the silica via siloxane bonds. This produces a material that, while appearing identical to the precursor Silica, is very hydrophobic, repels water and does not absorb moisture from the environment.
“Colloidal” is used in reference to both pyrogenic and precipitated silica. It may be confused with Silica colloids, which are also obtained via the wet chemical route. Note that the International Union of Pure and Applied Chemistry (IUPAC) defines colloids as systems (dispersions) in which particles of colloidal size (1 nm–1000 nm) of any nature (solid, liquid, or gas) are dispersed in a continuous phase of different composition or state.
Thus, in the strictest sense, the term ‘colloidal silica’ applies to stable dispersions (or sols) consisting of discrete particles of amorphous silica having sizes of between 5 and 100 nm. These colloidal silicas are commercially available in the form of sols or dried powders (e.g., xerogels, dry precipitates, aerogels, or calcinated coarcervates). In a broad sense, however, many other forms of silica (other than wet or dry silica sols above) are colloidal on the grounds that they are composed of particles in a colloidal state of subdivision (1-1000 nm).
It is also worth noting that the silica particles and aggregates are self-supporting and stabilised dispersions of silica particles in a continuous air phase and are unaffected by gravitational forces. Finally, fumed silica is commonly referred to as ‘colloidal’ because the silica powders are made by condensing a silica precursor from a vapour phase. In this sense, fumed silica particles are dispersed in a gas during its production process.
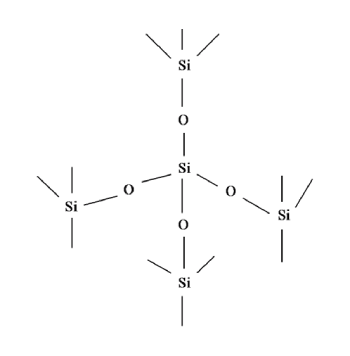
The SiO2 molecules in synthetic silicon dioxide do not exist in isolation. While the primary structure is the tetrahedron, consisting of one silicon atom bonded to four oxygen atoms, tetrahedrons arrange into networks. During the synthesis process, minute droplets of SiO2 initially aggregate into so-called nuclides, which combine stochastically into even larger particles, facilitated by weak physical interactions such as van der Waal’s forces.
Another important property of synthetic silica is its specific surface area. Fumed silicon dioxide, in particular, has only one surface, which is external and little or non-existent internal pore volume. Precipitated silica, on the other hand, is mesoporous and exhibits an internal surface. Generally, the higher the specific surface area, the greater the degree of agglomeration. It is these aggregates that partly contribute to the unique functionalities of amorphous silicon dioxide.
| Chemical Name | Silicon dioxide, chemically synthesised |
| CAS Registration Number | [112926-00-8] |
| Empirical Formula | SiO2 |
| Molecular weight | 60.08 |
| EC Number | 231-545-4 |
| UNII Code (FDA) | ETJ7Z6XBU4 |
Hydrated colloidal silicon dioxide is approved for use in pharmaceutical products and is monographed in the USP, Ph.Eur, JP and IP. It is also GRAS listed and included in the FDA Inactive Ingredients Database. Hydrated colloidal silicon dioxide is also approved by the FDA as a food additive and for food Contact. It is similarly approved in Europe and designated E551.
| Physical form | Solid, powder |
| Appearance | White |
| Flowability | Fluffy, poorly flowing or free-flowing powder |
| True Density | 2.2 g/c.c |
| Porosity | >0.03 |
| Pore size distribution | Very wide |
| DBP absorption | 80-320 |
| Hygroscopicity | Hydrated silica is hygroscopic. Moisture content is dependent on grade and storage conditions |
| pH value | 5.0-10.0 |
| Tapped Density | 30-500 g/l. (SIDENT® 22 S = 90 g/l; SIPERNAT® 50 S = 180 g/l) |
| Melting point | 17000C |
| Particle size distribution | Primary particle size in nano range (5 – 20 nm). However, Silica exists in the form of loose agglomerates of 1 to ≥300µm. |
| Refractive index | not available |
| Solubility | Practically insoluble in water, propylene glycol, glycerol, organic solvents, mineral oil, and most acids. Soluble in hot solutions of alkali hydroxide. |
| Relative density | 1.9-2.2 |
| BET Specific surface area | 100 – 600 m2/g depending on grade |
| USP-NF | Ph.Eur | |
| Name | Silicon Dioxide | Silica, Colloidal Hydrated |
| Authorised Uses | Excipient | Excipient |
| Definition | specified | specified |
| Characters | specified | specified |
| Identification | specified | specified |
| pH | 4.0-7.0 | 4.0-8.0 |
| Water absorption capacity | specified | n/a |
| Substances soluble in HCl | specified | ≤2.0% |
| Chlorides | ≤0.1% | ≤0.1% |
| Sulphates | ≤0.5% | ≤1.0% |
| Iron | n/a | ≤300 ppm |
| Arsenic | ≤ 3.0 ppm | n/a |
| Heavy Metals | 0.003% | ≤ 25 ppm |
| Loss on Ignition (%) | ≤ 8.5% | ≤ 20.0% |
| Loss on Drying (%) | ≤ 5.0% | ≤ 2.0% |
| TAMC | specified | specified |
| Gram -ve organisms | specified | specified |
| E.coli | specified | specified |
| Salmonella | specified | specified |
| Assay (%) | ≥99.0 | 98.0-100.5 |
| Labelling | specified | n/a |
Key: n/a = specification is not listed
No express or implied warranty is made regarding the accuracy of the information presented or specific properties or fitness for any particular purpose. This information is not intended to be a substitute for official compendia or monographs.
Similar to the anhydrous colloidal silicon dioxide, precipitated colloidal silicone is used in pharmaceuticals, cosmetics, and food products as a carrier, absorbent, rheology modifier, anticaking agent; clarifying agent; and grinding aid. It combines a high absorption capacity with good flowability making it the most suitable grade for applications that require carrier silica to convert liquids or deliquescent formulations into free-flowing powders.
In the pharmaceutical industry, Hydrated silica is used as a grinding aid for low molecular weight actives, as a granulation aid in weight granulation and to improve the wettability of powders (for instance, dry suspensions and oral powders). It can also be used to aid flowability.
In personal care and topical products, specific grades are used to improve the sensory effects/mattifying properties of the preparation. Occasionally, it can be used to improve the rheology/thixotropy of gels and emulsions.
| Application | Level (%) |
| Improve flowability/anticaking agent | 0.2 – 0.2 |
| Improve wettability of powders | Up to 10% |
| Granulation aid | Up to 10% |
| Carrier in topical products | 1.0 – 10.0% |
Colloidal silicon dioxide is widely used in oral and topical pharmaceutical products arid are generally regarded as an essentially nontoxic and nonirritant excipient. However, parenteral use (injection may produce local tissue reactions and/or granulomas). Colloidal silicon dioxide should therefore not be administered parenterally.
Toxicology: LD50 (rat, IV): 0.015g/kg; LD50 (rat, oral): 3.16 g/kg. Not a known carcinogen*
*Inhalation of Synthetic amorphous silicon dioxide dust can cause irritation of the respiratory tract membranes however it is not associated with fibrosis of the lungs (silicosis), which is well documented to occur when exposed to crystalline silica.
Hydrated colloidal silicon dioxide is hygroscopic and absorbs water from its environment. It should therefore be stored in a well-closed container. The assigned shelf life is a minimum of 24 months.
When handling the material in industrial settings, observance of applicable SHEQ protocols is recommended. Eye protection and gloves should be worn. Procedures that minimise dust production should be implemented, especially when handling large quantities of material.
Hydrated silica is considered a dust nuisance. Precautions should therefore be in place to minimise inhalation hazards. A dust mask should be worn even when processing small quantities of the material. For larger quantities, more robust procedures, such as a dust respirator, are recommended.
Hydrated silicon dioxide is a synthetic substance made from two materials that are abundant in nature, namely silicon and oxygen. It is a versatile material that is used in a wide range of industries. However, its production uses huge amounts of energy across the entire value chain, although not to the same degree as the Anhydrous grade. The Excipients Forum assigned it a Sustainability Score of 48 out of 100 under their Sustainable Chemistry™ scheme.
[1] Evonik Industries, AEROSIL(R) Pharma Colloidal silicon dioxide, Technical information.
[2] Evonik Industries, SIPERNAT and AEROSIL – an essential in industrial powder technology, Technical information.
[3] World Health Organization & Food and Agriculture Organization of the United Nations. (1991). Evaluation of certain food additives and contaminants : thirty-seventh report of the Joint FAO/WHO Expert Committee on Food Additives [meeting held in Geneva from 5 to 14 June 1990], (1991).
[5] G.K. Bolhuis, N. Anthony Armstrong, Excipients for direct compaction—an update, Pharmaceutical Development and Technology, 11 (2006) 111-124.
[6] L.C. Becker, W.F. Bergfeld, D.V. Belsito, R.A. Hill, C.D. Klaassen, D. Liebler, J.G. Marks, R.C. Shank, T.J. Slaga, P.W. Snyder, F.A. Andersen, Safety Assessment of Silylates and Surface-Modified Siloxysilicates, international Journal of Toxicology, 32 (2013).
[7] D. Majerová, L. Kulaviak, M. Růžička, F. Štěpánek, P. Zámostný, Effect of colloidal silica on rheological properties of common pharmaceutical excipients, European Journal of Pharmaceutics and Biopharmaceutics, 106 (2016) 2-8.
[8] Sccs, P.H.M. Hoet, Opinion of the Scientific Committee on Consumer Safety (SCCS) – Revision of the opinion on the safety of the use of Silica, Hydrated Silica, and Silica Surface Modified with Alkyl Silylates (nano form) in cosmetic products, regulatory toxicology and pharmacology, 74 (2016) 79-80.
[9] T.U.S.P. Convention, Food Chemicals Codex (11th Edition), (2018).
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.