As a pharmaceutical product formulator, you may have wondered what Disodium Edetate (also known as Sodium EDTA) is and how you can use it in your products. Given its ubiquity in pharmaceutical and other processing industries, these are not trivial questions, so kudos for wanting to familiarize yourself with its applications and limitations.
In simple terms, it is ethylenediaminetetraacetate as the disodium salt. It occurs as a white crystalline, odorless powder with a slightly acidic taste. According to the European Pharmacopoeia the pharmaceutical-grade material is required to contain NLT 99.0% and NMT 101.0% of C10H14N2Na2O8, calculated on a dried basis.
Chemical and physical particulars
Chemical Name: Ethylenediaminetetracetic acid, disodium salt
CAS Registry Number: [139-33-3]
Molecular weight: 336.2
Chemical formula and structure: [CH2N(CH2CO2H]2]2 Na2
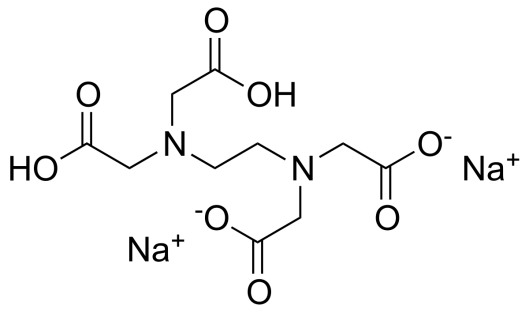
Acidity/alkalinity: pH 4.3 – 4.7 (1% w/v solution)
Solubility: Soluble in water (1 in 11 parts)
Current Regulatory Status
Currently listed in the Ph.Eur, USP-NF, BP and JP. It is also GRAS listed and included in the FDA Inactive Ingredients Database (Inhalations, Injections, Ophthalmic preparations, Oral capsules, Solutions, Suspensions, Syrups and Tablets, Rectal, Topical and Vaginal preparations).
Dsodium edetate is poorly absorbed from the gastrointestinal tract and associated with few adverse effects when used as an excipient. In larger doses, however, disodium edetate (and other edetates) cause calcium depletion (hypocalcemia), especially when used over an extended period of time.
However, this material should he used with caution in patients with renal impairment, tuberculosis, and impaired cardiac function. The WHO has set an estimated acceptable daily intake in foodstuffs of up to 2.5 mg/kg body-weight.
Uses in Pharmaceutical Formulations
Disodium edetate is used in topical, oral, ophthalmic and parenteral pharmaceutical formulations as well as in cosmetic and food products as a chelating and sequestering agent of heavy metal cations. The typical concentrations are between 0.005 and 0.1% w/v.
Chelation and sequestration of metal cations helps to stabilise, clarify and protect a formulation in many different ways. For instance, Edetate enhances the action of preservatives and stabilizes antioxidants; stabilises and protects polymeric thickeners, colorants, flavours and fragrances; and prolongs action of antioxidants such Tocopherol and ascorbate, for instance, in fats and oils.
Therefore, add edetate disodium (or other suitable edentates) to products that have a flavour, fragrance, colorant, polymeric thickener e.g carbomer, hyaluronic acid and hydrocolloid gums, preservatives, antioxidants, antibiotics, local anaesthetics and certain vaccines.
Disodium edetate is also used therapeutically as an anticoagulant due to its ability to chelate calcium and prevent the coagulation of blood in vitro.
Useful Tips and Comments
Edetate salts are more stable than EDTA. However, disodium edetate dihydrate loses water of crystallization when heated to 120o C.
Disodium edetate is hygroscopic and is unstable when exposed to moisture. The stability of the metal—edetate complex is dependent on the metal ion involved and the pH.
Disodium edetate is incompatible with strong oxidizing agents, strong bases and metal ions.
Manufacturers/Suppliers
Merck (Titriplex® II – EMPROVE® EXPERT PhEur, BP, USP, JP)
For a full description of this material, navigate to Disodium Edatate Monograph page.

