*Bricklane Innovations
St Johns Innovation Centre
Milton, Cambridge CB4 0WS
**Department of Pharmaceutics
UCL School of Pharmacy, University of London
29-39 Brunswick Square
London WC1N 1AX
Abstract
This study investigated the moisture barrier performance of polymer film coatings on a low hygroscopic tablet formulation based on dibasic calcium phosphate dihydrate and aspirin. Tablets were prepared by direct compaction and coated with aqueous dispersions of Eudragit® L30 D-55, Eudragit® EPO, Opadry® AMB and Sepifilm® LP.
Moisture uptake was studied by dynamic vapour sorption at 0 and 75% RH (25 oC). Stability was studied at 75 %RH/25 oC for 120 days and HPLC assay used to determine aspirin content.
Uncoated tablet cores equilibrated rapidly and took up very little water (0.11±0.006 %), confirming their low hygroscopicity. The amounts for coated cores varied as follows: 0.19±0.001 (Eudragit L30 D-55), 0.35±0.005 (Opadry AMB), 0.49±0.006 (Sepifilm LP) and 0.75±0.008 (Eudragit EPO) indicating that coated cores had higher uptake.
There was a progressive decrease in the strength of aspirin in all the samples studied, with the coated cores showing more pronounced degradation of the active (mean % aspirin recovered after 4 months was 80.02±0.04 for uncoated cores compared with 78.75±0.30 for cores coated with Eudragit L30 D-55, 76.15±0.55 for Opadry AMB, 75.98±1.25 for Sepifilm LP and 66.45±1.13 for Eudragit EPO).
It is concluded that the benefits of using polymer films as moisture barrier coatings to increase drug stability in tablet formulations of low hygroscopicity are limited.
1 Introduction
Many physical and chemical properties of pharmaceutical substances are modified when they take up appreciable amounts of water (Dawoodbhai and Rhodes, 1989). During pre-manufacture processing, raw materials may come into contact with water, and some moisture may be retained as a result. Finished products can also become exposed to water vapour during manufacture, for instance while in temporary storage in the warehouse before packaging or even when in use by the patient. Water sorbed this way has the potential to alter functionality of the product, including key properties like disintegration and dissolution, and chemical and physical stability (Carstensen, 1988). For many drug products, especially those with moisture-sensitive ingredients, preventing water uptake is a key objective.
There are many ways of minimizing water uptake into dosage forms and/or preventing its interaction with active drug substances susceptible to hydrolysis. One approach involves the careful selection of excipients that are able to bind or repel water. When combined with a variety of technologies and packaging solutions, the deleterious effects of environmental water can be mitigated (Zografi and Kontny, 1986; Ahlneck and Zografi, 1990; Alvarez-Lorenzo et al, 2000).
A relatively recent innovation is the application of polymer film coatings with moisture barrier properties onto actual unit solid dosage forms (Mwesigwa et al., 2005). This approach is attractive since it provides a means of limiting moisture uptake into the product in addition to the usual benefits associated with application of polymer film coatings.
An ideal moisture barrier coating should exhibit low permeability to water vapour. Additionally, the coating should have a high moisture binding capacity so that any sorbed water can be prevented from reaching into the core.
In previous studies, the moisture uptake and permeability characteristics of polymer films commonly used as moisture barrier coatings were described (Eudragit L30 D-55, Eudragit EPO, Opadry AMB and Sepifilm LP).
These studies showed that polymer films exhibited complex moisture sorption behaviour with no discernable differentiation of permeability characteristics on the basis of hygroscopicity. Crucially for moisture barriers, there was no relationship between either sorption or permeability characteristics of cast films and functionality as protective coatings after application onto hygroscopic tablet cores. Our studies found that the levels of aspirin degradation were inexplicably higher in the coated cores than the uncoated cores despite coated samples achieving a net reduction in moisture uptake.
The purpose of the current study therefore was to investigate whether the same moisture barrier polymer films applied onto a non-hygroscopic tablet core might offer better protection and ensure the stability of a hydrolysable active drug substance.
2 Materials and Methods
2.1 Materials
Poly(methacrylic acid ethyl acrylate) copolymer (Eudragit L30 D-55, Evonik, Darmstadt, Germany), poly(butyl methacrylate (2-dimethylaminoethyl) methacrylate methyl methacrylate copolymer (Eudragit EPO, Evonik), a polyvinyl alcohol (PVA) – based coating system (Opadry AMB, Colorcon, Dartford, UK); and a hypromellose-based coating system (Sepifilm LP 014, Seppic, Paris, France) were free samples from the respective vendors.
Dibasic calcium phosphate dihydrate (Emcompress, JRS Pharma, Rosenberg, Germany) was purchased from JRS Pharma. Aspirin (USP Grade), triethyl citrate, talc, titanium dioxide, poly ethylene glycol (PEG) 6000, stearic acid, magnesium stearate, sodium lauryl sulphate, and carboxy methylcellulose sodium were all purchased from Sigma Aldrich (Dorset, UK).
2.2 Methods
2.2.1 Tablet Preparation and Coating
Tablet cores were obtained by direct compression and were based on aspirin (30%), dibasic calcium phosphate dihydrate (69.5%) and stearic acid (0.5%) using an eccentric tablet press (Manesty, Merseyside, UK). The tablet target weight was 200 mg and a breaking force strength of ≥ 70N.
Tablet core coating was undertaken in a laboratory-scale fluidized bed coater (Aeromatic-Fielder AG, Switzerland) at 40 oC. The coatings vendors’ recommended guidelines were followed to achieve theoretical dry weight gains of 1.8 % (Eudragit L30 D-55), 6.4% (Eudragit EPO), 4% (Opadry AMB), and 3 % (Sepifilm LP).
All coated and uncoated samples cores were thoroughly dried in a vacuum oven (for six hours at 40 oC) and stored in closed bottles over phosphorous pentoxide dessicant pending further tests.
The aspirin activity remaining in the tablet cores after coating application was compared with that of uncoated tablets. The degradation in coated samples was found to be less than 0.1 %.
2.2.2 Equilibrium Moisture Sorption-Desorption Studies
Moisture sorption characteristics of uncoated and coated tablet cores were studied in a dynamic vapour sorption apparatus (DVS 1, Surface Measurement Systems, London, UK).
Relative humidity (RH) was programmed to expose samples at 0% RH and then automatically switch to 75 %RH. The equilibration condition for each RH stage was set at a mass change rate of 0.001 %/min between two consecutive measurements.
All experiments were performed in triplicate at 25 oC. The results of water uptake are reported as the per cent dry basis (db) versus exposure time.
2.2.3 Aspirin Stability Studies
Stability studies were undertaken at 75% RH/25 oC in sealed glass desiccators, which were placed in a thermostatted incubator (Sanyo-Gallenkamp, Loughborough, UK). The RH condition was provided by a NaCl slurry. Sampling was undertaken periodically once a month for a total period of four months.
2.2.4 HPLC Assay
A previously validated HPLC method (Fogel et al, 1984) was used (with minor modification) with to assay the retained strength of aspirin with the tablets. The HPLC conditions were an integrated HP 1050 Series HPLC system, an Agilent Zorbax Exclipse XDB–C8 4.6×150 mm column and a water-acetonitrile system (75:25) acidified with orthophosphoric acid as the mobile phase.
3 Results and Discussion
The specific equilibrium moisture uptake data at the 0-90% RH/25C cycle for uncoated and coated tablet cores are shown in Fig. 1. We elected to use this cycle to illustrate the sorption patterns otherwise the rest of the data and analysis are based on the 0-75%/25C cycles.
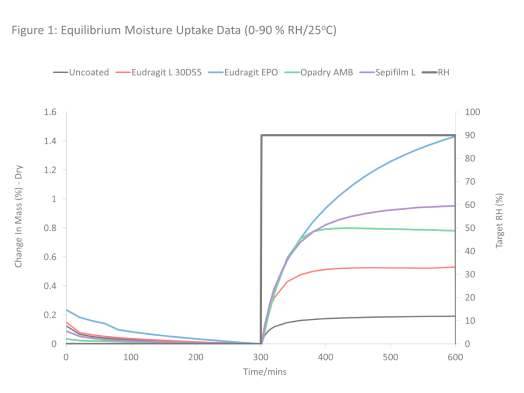
The data show that uncoated cores equilibrated rapidly (within 50 min) and took up very little water (0.11 ±0.006 % dry basis). When contrasted with hygroscopic cores under the same studied (reported elsewhere Mwesigwa et al, 2008) showed that the uptake at 75% RH was 2.91 ± 0.011 % and equilibration time was 500 min. This therefore demonstrates that the sorptive properties of non-hygroscopic cores used in this study were limited, confirming the non-hygroscopicity of the excipients used in the formulation.
With respect to data for coated low-hygroscopic cores, it is clear that coated cores sorbed considerably more water than the uncoated samples and also took longer to equilibrate. There were further differences in sorption patterns of the different coatings. For example, Eudragit L30 D-55 and Sepifilm LP coated cores exhibited nearly similar uptake profiles (similar rates of mass change) but the total amounts of water sorbed were different. These differences are best illustrated in Figure 2, which shows the specific equilibrium mositure uptake at each time point.
The cores coated with Opadry AMB and Eudragit EPO exhibited slower rates of uptake. However, the Eudragit EPO coated core took longer to equilibrate and also sorbed the most amount of water (0.75 ± 0.008%). These results appear to suggest that application of the barrier coatings to non-hygroscopic cores did not slow the moisture uptake kinetics and may actually have “enhanced” uptake into the core. In effect, the moisture uptake characteristics obtained are from the applied films rather than the cores, the former being the more hygroscopic component.
This contrasts with the behaviour of hygroscopic cores previously reported, where it was observed that applied films resulted in a marked reduction in the water uptake and therefore thought to have contributed less to the sorption equilibrium of the cores.
Nevertheless, it should be noted that even with this apparent “enhancement”, the equilibrium total water uptake of coated non-hygroscopic cores was still only a fraction of that observed for the hygroscopic formulation (e.g., the equilibrium uptake at 75% RH for the hygroscopic core coated with Sepifilm LP was 2.18 ± 0.001 compared with 0.49 ± 0.060 for the non-hygroscopic core coated with the same polymer).
The distribution of water between the core and the applied film is an important performance characteristic of a barrier system. To determine the availability of sorbed moisture in the applied films and the tablet cores, it is necessary to recap the equilibrium moisture sorption and permeability data obtained with free standing cast films in our earlier report (Mwesigwa et al, 2008) together with the data in Fig. 1. The data showed that at 75% RH Eudragit EPO free film was the least hygroscopic with an equilibrium moisture uptake of 1.85 ± 0.255 %. The Eudragit L30 D-55 took up 2.59 ± 0.195 % db); followed by Opadry AMB (5.18 ± 1.169) and Sepifilm LP (9.64 ± 0.252 %).
In terms of permeability, the best barrier films were Eudragit EPO and Opadry AMB (permeability coefficients at 75% RH were 0.58 x10-6 and 0.69 x10-6 cm3 (STP) cm/cm2s.cmHg, respectively). Eudragit L30 D55 and Sepifilm LP free films with permeability coefficients of 1.58×10-6 and 1.92 x10-6, respectively. The data for the equilibrium sorption (in Fig. 1) provides an interesting contrast; for instance, it can be seen that cores coated with Eudragit L30 D-55 were the least hygroscopic, followed by Opadry AMB, Sepifilm and Eudragit EPO.
This suggests that the barrier properties of the films were not being replicated on the cores. This pattern becomes more clear when the distribution of the sorbed water between the film and the core is considered.
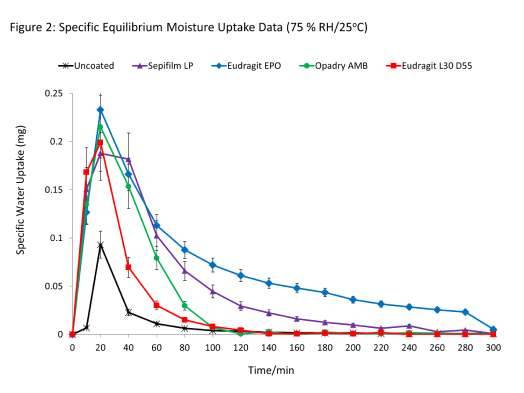
Table 1 displays the amounts (in mg) of moisture in the applied coatings (calculated from the product of the total uptake in % of the cast film and the weight in mg of applied coating) and the total water uptake (in mg) of the coated sample (being the product of % total water uptake and the weight in mg of coated sample divided by 100), and from which the amount of water that potentially reached the core can be easily obtained (being the difference of the two quantities). It can be seen that the Eudragit EPO coated samples had the highest amount of water in the core despite the low hygroscopicity and permeability of the free film. Eudragit L30 D-55 and Opadry AMB coated samples had the least quantities of water sorbed in the cores.
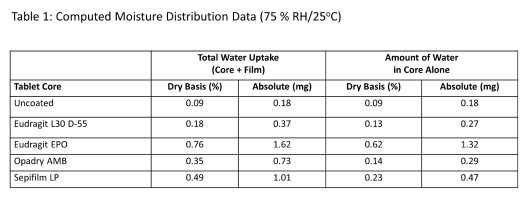
The behaviour of Opadry AMB system is of interest: the free film exhibited comparable permeability to that of Eudragit EPO but had much higher hygrocopicity, on the core, this film showed lower levels of water ingress into the core, which were comparable to Eudragit L30 D-55. Therefore, as in our previous report, there appears to be no meaningful relationship that can be established between either water sorption or permeability characteristics of free films and the protective function of the coatings on non-hygroscopic tablet cores.
When a coated tablet is exposed to moisture stress, it is expected that the applied coating will either completely repel the moisture or hold a portion of the moisture and allow the excess into the underlying core depending on its permeability and the amount that the core can accommodate at the prevailing conditions. Equilibrium sorption sorption is achieved when the water activity of the sample equals that of the surroundings.
Given that the cores have lower water attracting propensity than the coatings, the sorption equilibrium is largely directed by the more hygroscopic films. Under these conditions, the cores easily reach their maximum water holding capacity and any extra sorption above this amount is taken up by the film to the point equilibration with the surrounding RH is achieved. We propose this is the reason for the observed failure of the coatings to achieve a net reduction in the total amount of water taken up over the uncoated cores. This also accounts for the observation that total sorption of low hygroscopic cores are nearly a simple sum-total of that taken up by the core and the film.
Do the above results have any bearing on stability given the relatively low water uptake in non-hygroscopic cores? Figure 3 shows the results of the stability studies of aspirin in uncoated and coated tablet cores.
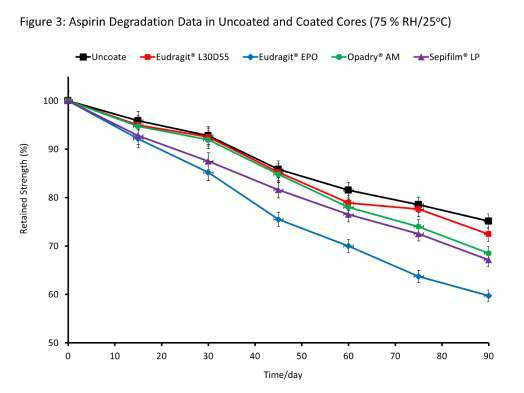
Firstly, it is apparent, just like the data for hygroscopic cores, that there was a progressive decrease in the strength of aspirin upon exposure to moisture stress in all samples. By the end of study, uncoated cores exhibited the lowest levels of aspirin degradation. It is worthy emphasising at this stage that the degradation observed in the samples was solely attributed to moisture uptake post coating in the fluidised bed coater.
As it would normally be expected that samples exhibiting the highest water uptake also show the greater aspirin hydrolysis, the outcome of the stability studies does not appear paradoxical. However, when viewed in the context of the barrier characteristics of the films or the total amount of water taken in (and in comparison to the hygroscopic cores), it can be seen that despite the non-hygroscopic cores taking up only a fraction of the amount of water taken up by the hygroscopic cores, the extent of degradation was higher in these samples. Also, as no correlation between water sorption/permeability characteristics of free standing films, total water uptake of uncoated/coated cores with the extents of hydrolysis of aspirin in the cores to which the films have been applied can be established, it would suggest that the ability to prevent drug degradation in the non-hygroscopic tablet cores is not necessarily the result of preventing moisture uptake, per se.
It is already well known that excipients play an important role in the stability of moisture sensitive actives. For instance, the influence of the particle size and pore volume distribution of dibasic calcium phosphate dihydrate on the hydrolysis of aspirin has been reported (Landin et al, 1994).
Other factors, including hardness (Lee et al., 1966); excipient type and crystallinity (Maulding and Zoglio, 1969; Ahlneck and Alderborn, 1988, Du and Hoag, 2001) have been discussed. In this present study, we found that the low-hygroscopic formulation exhibited greater degradation despite the lower levels of water uptake. In this formulation, more of the sorbed moisture was available as free water, which was more available to interact with aspirin.
It is also apparent that while coated cores sorb more water than the uncoated cores, we are inclined to believe that considerably higher levels of degradation of coated non-hygroscopic cores are a result of a combination of other factors rather than water uptake alone. We have previously proposed that when coated tablets are exposed to moisture stress, the adhesion of the coating to the core is compromised and this augments the degradation of aspirin over and above the levels that would be observed through simple permeation into the core (Mwesigwa et al, 2008).
There is already a significant body of corroborating evidence to support our proposition (Okhamafe and York, 1985, Felton and McGinity, 1997). It is widely known that adhesion is of primary importance to barrier performance and its loss compromises the ability of the coating to provide mechanical protection to the substrate (Fung and Parrot, 1980, Klages et al 1996 and Barranco et al, 2004), who have reported the formation of a water layer at a paint film-substrate boundary and associated this phenomenon with a reduction in adhesion to the substrate and greater levels of corrosion in oxidation.
4 Conclusions
This study was undertaken to understand whether the stability of a moisture sensitive active could be improved through application of moisture barrier coatings on tablet cores exhibiting low hygroscopicity. The moisture barrier coatings applied to tablet cores did not achieve a net reduction in the amount of moisture sorbed when exposed to elevated RH. The uncoated cores exhibited lower overall levels of aspirin degradation compared with coated samples. The use of current moisture barrier coatings on tablet cores with minimal water uptake characteristics does not appear to prevent the degradation of moisture sensitive actives.
References
Ahlneck, C., Alderborn, G. 1988. Solid-state stability of acetylsalicylic-acid in binary-mixtures with microcrystalline and microfine cellulose, Acta Pharm. Suec. 25, 41–52.
Ahlneck, C., Zografi, G. 1990. The molecular basis of moisture effects on the physical and chemical stability of drugs in the solid state. Int. J. Pharm. 62, 87-95.
Alvarez-Lorenzo, C., Gomez-Amoza, L., Martinez-Pacheco, R., Sonto, C., Cocheiro, A. 2000. Interactions between hydroxypropyl celluloses and vapour/liquid water. Eur. J. Pharm. Biopharm. 50 (2000) 307-318.
Barranco, V. Carpentier, J. Grundmeier G. 2004. Correlation of morphology and barrier properties of thin microwave plasma polymer films on metal substrates. Electrochim Acta 49, 1999-2013.
Buckton, G., Darcy, P. 1996. Water mobility in amorphous lactose below and close to the glass transition temperature, Int. J. Pharm. 136, 141–146.
Carstensen, J.T., 1988. Effect of moisture on the stability of solid dosage forms. Drug Dev. Ind. Pharm. 14, 1927-1969.
Dawoodbhai, S., Rhodes, C.T., 1989. The effect of moisture on powder flow and on compaction and physical stability of tablets. Drug Dev. Ind. Pharm. 15, 1577-1600.
Du, J., Hoag, S.W. 2001. The influence of excipients on the stability of the moisture sensitive drugs aspirin and niacinamide: comparison of tablets containing lactose monohydrate with tablets containing anhydrous lactose, Pharm. Dev. Technol. 6, 59–66.
Felton, L.A., McGinity. J. W. 1997. Influence of plasticizers on the adhesive properties of an acrylic resin copolymer to hydrophilic and hydrophobic tablet compacts. Int J Pharm 154, 167-178.
Fogel, J., Epstein, P., Chen, P. 1984. Simultaneous high-performance liquid chromatography assay of acetylsalicylic acid and salicylic acid in film-coated aspirin tablets. J Chromatog A 327, 507-511.
Fung, R.M., Parrot E.L. 1980. Measurement of film-coating adhesiveness. J Pharm Sci 69, 439-441.
Klages, C.P., Dietz, A, Höing, T. Thyen, R., Webber, A, Willich, P. 1996. Deposition and properties of carbon-based amorphous protective coatings. Surf Coat Technol 80, 121-128.
Landín, M., Perezmarcos, B., Casalderrey, M., Martinez-Pacheco, R., Gomez-Amoza, J.L., Souto, C., Concheiro A., Rowe, R.C. 1994. Chemical-stability of acetylsalicylic-acid in tablets prepared with different commercial brands of dicalcium phosphate dihydrate, Int. J. Pharm. 107, 247–249.
Lee, S., De Kay, H.E. Banker, G.S. 1966. Effect of water vapour pressure on moisture sorption and stability of aspirin and ascorbic acid in tablet matrices. J. Pharm. Sci. 54, 1153-1158.
Maulding, H.V., Zoglio, M.A., 1969. Pharmaceutical heterogeneous system: IV. A kinetic approach to the stability screening of solid dosage forms containing aspirin. J. Pharm. Sci. 58, 1359–1362.
Mwesigwa, E., Basit, A.W, Buckton, G. 2008. Moisture sorption and permeability characteristics of polymer films: Implications for their use as barrier coatings for solid dosage forms containing hydrolysable drug substances, J. Pharm. Sci. 97, 4433-4445.
Mwesigwa, E., Buckton, G., Basit, A.W 2005. The hygroscopicity of moisture barrier film coatings, Drug Devt. Ind. Pharm. 10 959-968.
Okhamafe, A.O., York, P. 1985. The adhesion characteristics of some pigmented and unpigmented aqueous-based film coatings applied to aspirin tablets. J Pharm Pharmacol 37, 849-853.
Zografi, G. 1988. States of water associated with solids. Drug Dev. Ind. Pharm. 14, 1905-1919.
Zografi, G., Kontny, M.J., 1986. The interaction of water with cellulose and starch-derived pharmaceutical excipients. Pharm. Res. 3, 187-194.