A day in the life of a Biotech start-up founder
As part of a new series, we’ll interviewing different people in the biopharmaceutical R&D space – including scientists, founders and noteworthy leaders – to show what day-to-day life looks like for them.
To kick this off, we recently interviewed Dr.Alvaro Goyanes (pictured), Co-founder and Development Director of London-based FabRx Ltd., a specialist biotech company at the forefront of 3D printing technologies and personalised medicines.
Please tell our readers about yourself
I am Dr Alvaro Goyanes and my current role at FabRx is Development Director and Lead Project Researcher. I am a pharmacist by training, I hold a PhD in pharmaceutical technology from University of Santiago de Compostela (Spain) and I have been at University College London (UCL) – School of Pharmacy as a researcher and as a honorary lecturer for more than 8 years. I have over 10 years’ industry experience in research and academia.
How did the idea for FabRx come about?
I can’t say there was a specific light bulb moment. During my post-doctoral studies here at UCL School of Pharmacy, I was involved in the investigating the potential use of 3D printing to make medicines. It was then that we zeroed on using Fused Deposition Modeling (FDM) that we saw a potential business opportunity, and the idea grew from there.
Having set up FabRx Ltd, what does a typical day look like for you?
Firstly, there is no such thing as a typical day. However, there are things that I do most days. Typically, I start once I am done with my morning routine (exercise, shower, coffee and time to read newspapers), I head off into my home office to get a head start on my emails before any other work starts. Typically, I may have 180 – 200 emails.
I may have business calls with the leadership team or investors, which I attend to first thing. Otherwise, my most important task is providing customer support for FabRx’s M3DIMAKER™ and the necessary communications side of things. Being responsible for development, I will be working to understand how they use our products and obtaining their suggestions as to how to improve. I may also pop into the laboratory to investigate something in relation to the development programme but this is dependent on other things going on.
Obviously, being a start-up company, a good part of my work is supporting the rest of the team with respect to the company’s long-term goals. A large part of this will be meeting all sorts of stakeholders, potential investors, customers, to build relationships and communicate our mission and what we are seeking to achieve.
And finally, I have internal meetings to support our team members in their roles and to feedback on proposed improvements to our products and research programmes, which is very critical to a start-up company such as ours.
Can you tell us more about M3DIMAKER™?
M3DIMAKER is the world’s first pharmaceutical printer in the market that we developed at FabRx. It is designed to prepare personalised medicines close to the patients, and it is mainly oriented to be placed in pharmacies and hospital, to prepare the medicines under GMP conditions. Since it was designed by us, it is specially adapted to print medicines safe and easy.

https://www.youtube.com/watch?v=404atdYX-nk&ab_channel=FabRx
What is the most challenging aspect of your job?
As you well know, for any initiative to succeed, company leaders must have a set of clear priorities to make it happen. I think the most challenging thing for me is prioritising every day and getting all issues aligned to the startup’s long-term mission. I find that there are always a million things I could be working on, be it R&D, communications, selling, motivating the research team, setting strategy, the press…. So figuring out what is worth my time and taking that decision not knowing the end outcome is not easy. As a start-up in a niche space, we do not have a playbook, which is great but also leads to a lot of decision fatigue in this respect.
And what about the most rewarding?
Without a doubt, the most rewarding part of the role is seeing the company grow and create an impact in patient’s lives. We really believe in personalised medicine and think that 3D printing is the best technology to reach it. But make no mistake, founding something is hard work, and there are some dark moments, but what lifts my spirit is when we get feedback from the doctors and users. This is worth all the hard work.
Were you always cut out to be an entrepreneur?
I do not pay attention to labels, such as entrepreneur this or that. What has always motivated me is to make a positive impact to my community and those I interact with daily.
What advice would you have for future and current founders?
Be prepared to wear a lot of different hats and face challenges.
You can find out more about FabRx and its products from www.FabRx.co.uk
Unlocking the Power and Versatility of Plant-Based Softgels
While plant-based diets continue to grow in popularity, the desire to live and act more sustainably and healthfully is on the rise.
Why are some COVID-19 vaccines working better for men than women?
If there’s one take-home message for the general public about the coronavirus vaccines approved in the U.S., it’s that they are remarkably effective.
Takeda announces approval of Moderna’s COVID-19 vaccine in Japan
AstraZeneca COVID-19 vaccine Vaxzevria authorised for emergency use in Japan
Carbomers: Overview, Key Properties and Formulating Tips
Carbomers are an important group of excipients that every well-meaning formulator should become familiar with. Here is a quick run-through of what they are, uses and formulation tips.
Chemistry and Physical Description
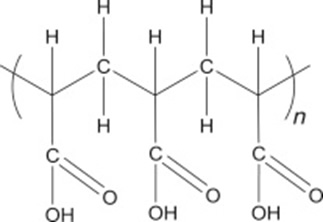
Carbomers are synthetic, chemically related, high molecular weight, nonlinear polymers based on crosslinked acrylic acid chemistry.
Originally developed by BF Goodrich and trademarked CARBOPOL® in 1958. These materials (especially, Carbopol 940, 941, and 934) revolutionised topical products by enabling formulators to create new types of product previously not possible.
The general chemical structure of carbomers is shown below:
Key Physicochemical Properties
- Molecular weight 700 kDa to 4 000 000 kDa
- Hygroscopic
- Powdered carbomers have a dry particle agglomerated size of 2-7µm.
- Do not dissolve but swell in ethanol, water, propylene glycol and glycerin to form microgels.
- Dispersions are acidic with a pH ~3. Upon neutralization (pH 7), particles swell to around 1000 times their initial volume and the viscosity dramatically increases due to charge repulsion.
- Can produce clear gels in water and ethanol due to refractive index matching.
- Highly crosslinked carbomers are commonly used as super absorbers in disposable diapers.
- Salts can decrease viscosity by reducing the charge repulsion.
Applications
- Carbomers are listed in the USP-NF, PhEur, BP; JP, IP and ChP.
- Grades with residual benzene content > 2 ppm do not meet the specifications of current pharmacopoeia monographs.
- Carbomers with low residuals of other solvents other than the ICH-defined Class 1 – 2 solvents may he used in Europe.
- Carbomers with low residuals of ethyl acetate, such as Carbopol 971P NF, are permitted for use in oral preparations, e.g suspensions, capsules or tablets.
- For topical products, carbomers can be used as gelling agents (0.1 – 2.0%), controlled-release agents (5 – 30.0%), emulsifying agents (0.5 – 1.0%), emulsion stabilizers (1.0%), rheology modifiers (0.5 – 1.0%) and stabilizing and suspending gents (0.5 – 1.0%).
- Carbomers are also employed as emulsifying agents in the preparation of oil-in-water emulsions for external administration.
- Carbomers can be used as bioadhesive polymers (0.1 – 0.5%), tablet binders (0.75 – 3.0%) and controlled release agents.
- Carbomers can aTopical medical devices (Ultrasound adhesive gel and personal and medical lubricants, and artificial tears)
Advantages
- Versatile and multifunctional excipients for oral (solid and liquids) and topical formulations.
- Synthetically derived, hence free from irregularities of natural products.
- Available in multiple grades and properties to meet different formulation or product performance requirements.
- Highly efficient thickeners at very low levels (<1% polymer). Suspensions and emulsions are efficiently stabilised due to the high yield value gels.
- Can make aqueous or alcoholic clear gels.
- Can make emulsifier free oil in water crème gel formulations.
- Can make stable water in oil in water emulsions.
- Excellent skin feel (<.5%) and shear thinning rheology.
Formulating Tips
Picture credits: Silverson FLASHBLEND Mixer
- Lightly cross-linked carbomers (lower viscosity) are more efficient at controlling drug release compared with highly cross-linked carbomers (higher viscosity).
- If used in wet granulation processes, water, solvents or their mixtures can be used as the granulating fluid. To control tackiness of the wet mass include talc in the formulation.
- Carbomers from different manufacturers or grades produced via different manufacturing processes may not have identical properties. Therefore, grades should not be interchanged without performance equivalency ascertainment.
- When preparing carbomer gels, powders should first be dispersed into vigorously stirred water, taking care to avoid the formation of agglomerates.
- The dispersion should then neutralized by the addition of a suitable base.
- Use granulated grades to reduce dusting issues during manufacturing.
- Carbomers can easily be added to emulsions by addition to the oil phase prior to emulsification.
- Adding electrolyte or small amounts of acid to the water phase prior to Carbomer addition significantly improves its dispersion by reducing solution viscosity. Up to 5% dispersions of Carbomer in water can typically be made with this approach.
- Agitation of the dispersion should be done carefully and gently with a broad, paddle-like stirrer to avoid introducing air bubbles.
- The viscosity of gels is significantly reduced at pH values less than 3 or greater 9, or in the presence of strong electrolytes.
- Suitable neutralising agents include amino acids, potassium hydroxide, sodium bicarbonate, sodium hydroxide, and organic amines such as triethanolamine.
- One gram of carbomer is neutralized by approximately 0.4 g of sodium hydroxide.
- A number of manufacturers have introduced grades to overcome the challenges of dispersing powders in aqueous solvents, e.g Lubrizol’s Carbopol Ultrez.
- Gels rapidly lose viscosity on exposure to UV light. To minimise this add a suitable antioxidant.
Leading Manufacturers of Carbomer Excipients
- Lubrizol
- Ashland
- Corel Pharma Chem
Recommended Carbomers for Pharmaceutical Formulations
- Ultrez 30 (Lubrizol) has been shown to exhibit better electrolyte tolerance than other grades of Carbomer.
- Ultrez 10 (Lubrizol) is a universal carbomer for broad applications. A 5% dispersion of Ultrez 10 exhibits viscosities in the 50 – 55 MPa s range.
Rogue antibodies wreak havoc in severe COVID-19 cases
The development of antibodies to the COVID-19 virus has been the great long-term hope of ending the pandemic. However, immune system turncoats are also major culprits in severe cases of COVID-19, Yale scientists report in the journal Nature.
These autoantibodies target and react with a person’s tissues or organs similar to ones that cause autoimmune diseases such as lupus or rheumatoid arthritis.
Acacia Gum and Bentonite
Acacia Gum and Bentonite
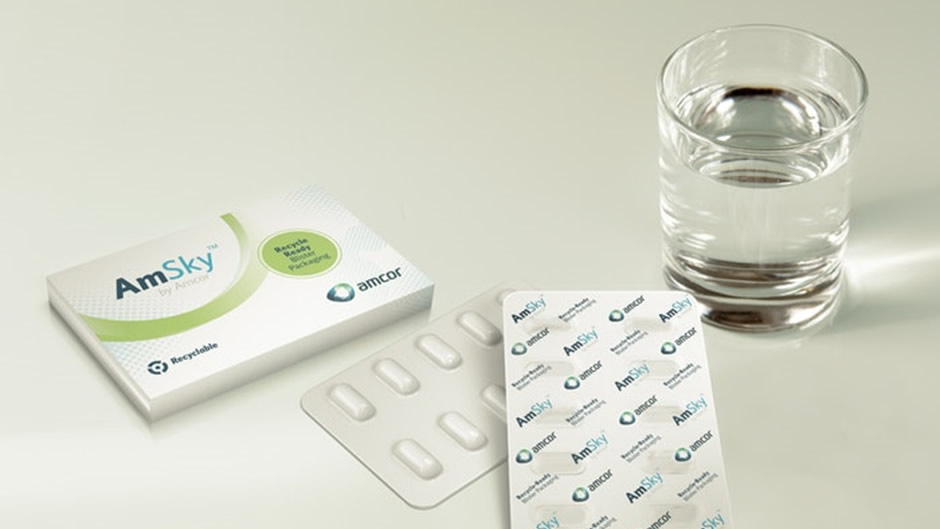
Amcor’s innovative recyclable blister packaging moves closer to commercialization AmSky blister system aims to eliminate PVC from blister packaging
Enabling pharmaceutical customers to improve packaging recyclability
Amcor, the Zurich-based packaging technology provider, announced on 29th April the start of customer trials of its highly anticipated recyclable polyethylene-based thermoform blister packaging, The new packaging is designed not only to meet the stringent standards of pharmaceutical packaging it also creates a more sustainable alternative for this highly demanded packaging type.
According to the company, AmSky™ achieves up to 70% reduction in carbon footprint, when compared to packaging alternatives on the market today. This is achieved by eliminating polyvinyl chloride and substituting it with a Polyethylene (PE) thermoform blister and lidding film.
The use of polyvinyl chloride makes attempts to recycle packaging more difficult. By removing it – while retaining all the benefits of pre-existing blister packaging – Amcor hopes to avail a more sustainable and environmentally friendly solution.
To speed up up development, Amcor has elected to co-develope and trial AmSky with a number several large pharmaceutical companies. It is expected that AmSky will be available in the second half of 2022.
 Peter Konieczny, Amcor’s Chief Commercial Officer, said: “Amcor is deploying our unique innovation capabilities to solve the biggest and most significant issues in packaging today. With AmSky Amcor has signalled our commitment to breakthrough innovation in the healthcare space.”
Peter Konieczny, Amcor’s Chief Commercial Officer, said: “Amcor is deploying our unique innovation capabilities to solve the biggest and most significant issues in packaging today. With AmSky Amcor has signalled our commitment to breakthrough innovation in the healthcare space.”
William Jackson, Amcor’s Chief Technology Officer for Flexibles, commented: “This exciting solution is a result of Amcor’s continued focus on advanced technology and growth, using the entire power of our global R&D network to bring recyclable solutions to our customers.”