Formulation of Medicated Chewing Gum: Excipient Handling Procedure to Prevent Problems
Medicated Chewing Gum Excipient
Chewing gum is popular among consumers, being enjoyed by people of all ages. The first successful formulation of medicated chewing gum products was in the early 1970s when Nicorette was launched by Pfizer for use in smoking cessation.
Since then, directly compressible chewing gum has quickly gained momentum as a readily adaptable platform for delivery pharmaceutical and nutracetical actives. it is currently recognized as a dosage form and defined in pharmacopoeias, including the European Pharmacopoeia.
Aside from their reported health benefits, such as helping boost blood flow to the brain and strengthening tooth enamel, chewing gums offer the following advantages to the pharmaceutical product formulator:
- Chewing gum can help increase patient adherence
- It offers fast onset of action and can improve bioavailability of drugs as some amount of drug is absorbed through the buccal mucosa
- It can also be administered discretely and without the need for water.
Although directly compressed gum excipient systems are widely promoted as relatively trouble-free and amenable on standard tableting equipment, product developers often experience several technical challenges during formulation and evaluation of medicated chewing gum products. This Technical Note provides a quick reference guide on how to handle and trouble-shoot common issues when using this material.
Types of Medicated Chewing Gum and Manufacturing Methods
There are currently two types of medicated chewing gum and manufacturing methods, namely extruded gum and compressed gum.
Extruded gums are produced using a traditional method of manufacture whereby the ingredients are intensively mixed at elevated temperature to produce a matrix. The matrix is then cut into ‘pillows’ and subsequently sprayed with coating agents.
Compressed gums are manufactured by blending active ingredients with the gum base and compressing the obtained mix on a tableting press. The direct compression method is a cool and dry process suitable for a wide range of ingredients. An example of a compressed gum system is the Health in Gum® excipient by CAFOSA SA, part of the MARS WRIGLEY conglomerate.
The Health in Gum® excipient system, the focus of this Technical Note, utilizes a food-grade gum base, polyols, plasticizers and anticaking agents. The active ingredient(s), sweeteners and flavours are added to the base, blended and compressed on standard tableting equipment. Additional processing or adaptations of equipment is not required.
Recommended Procedure for Handling Health in Gum®
- Break the lumps, if any, with a vibrating sieve (1 mm). Remove any pieces that you cannot break manually.
- In the selected blender, add the powder gum, the active and any other ingredient in powder form (e.g flavour in powder form, intensive sweeteners and colours) following the suggested formulation. Mixing time will depend on the type of mixer and the batch size. Gentle mixing needs more time but it tends to be better than intensive mixing.
- Add the liquid flavour so that it distributes homogeneously into the blend (for instance, spraying). If you cannot add the liquid to the mixer, you need to make a premix of the flavour and some of the silicon dioxide.
- After spraying, add between 0.5% of Silicon Dioxide with high oil absorption capacity (for instance, Sipernat 50S from Evonik or Syloid 244FP from Grace) and mix until you get a dry and free flowing.
- Add lubricant (2% on the case of Magnesium stearate) into the blender and mix until homogeneous.
- Sieve again, as some ingredients may agglomerate (liquid flavour and silicon dioxide).
- Before adding the final mix to the pressing machine, add a small quantity of the selected lubricant to the tablet press and run the machine slowly for a few minutes to allow the lubricant to cover punches. This will reduce the initial stickiness.
- Start compressing. Standard speeds are between 30k and 40k tablets/hr. Adjust pressure and weight, with final tablet hardness between 7 and 10 mm.
Tips and Suggestions
- Add silicon dioxide before adding the liquid flavour. The total silica content should however not exceed 2% to give the chewing gum the optimum texture.
- The liquid flavour should have Triacetin or a suitable oil (e.g Vitamine E) as the solvent to be chewing gum compatible
- Ensure that the product does not exceed 27ºC during processing. Heating up leads to a loss of fluidity and increases stickiness.
- Experiment with compression forces as they have different effects on gum texture depending on the formulation ingredients.
- Some ingredients with acid properties may affect to the texture of the gum. In this case, we suggest changing metallic stearates by stearic acid.
- Gums should be stored away from excessive heat, in a dry cool area. Keep away from direct light. The optimum conditions: 20ºC – 50% RH.
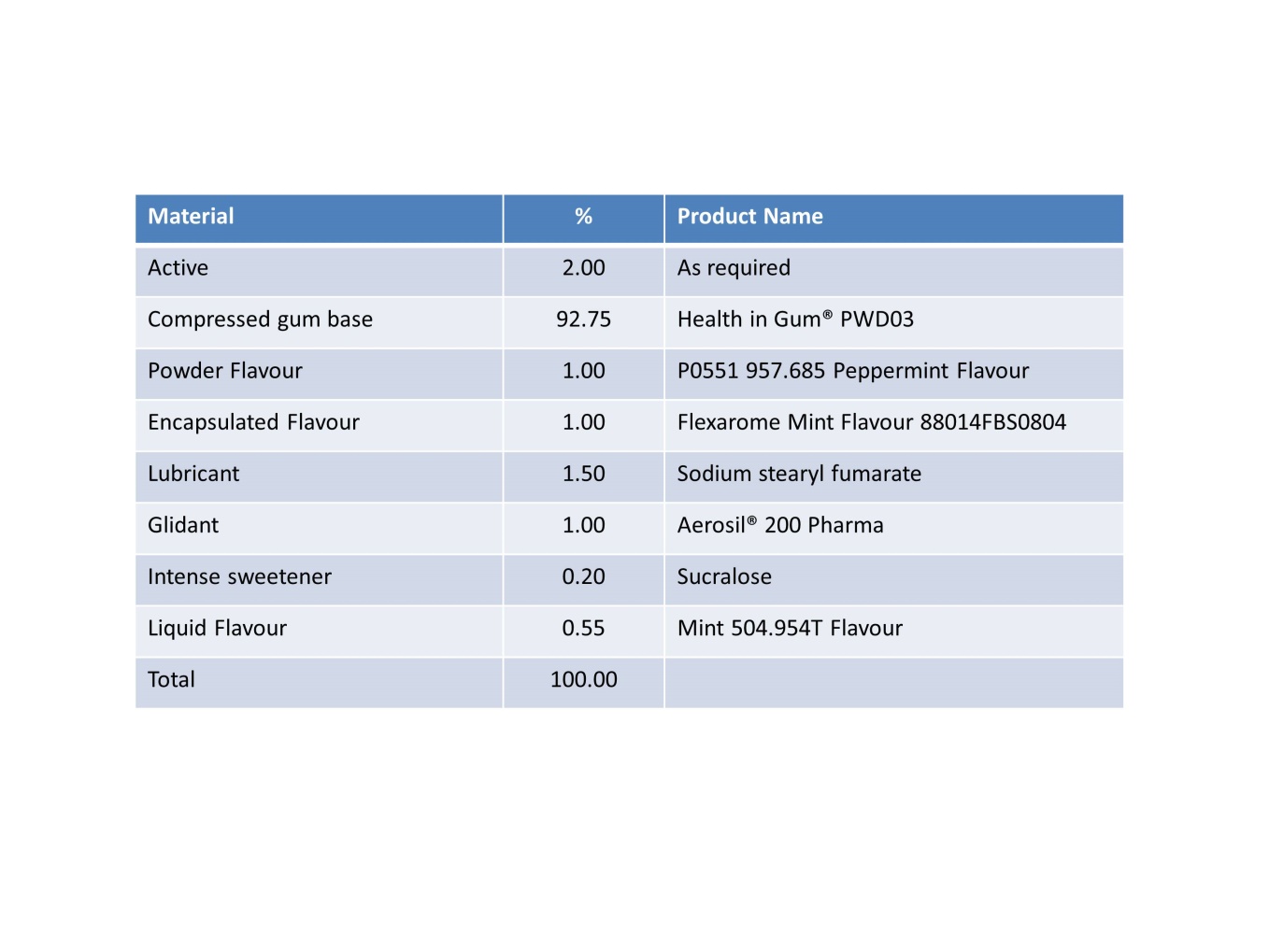
Suggested starting formula
Here is a simple formula to get you started:
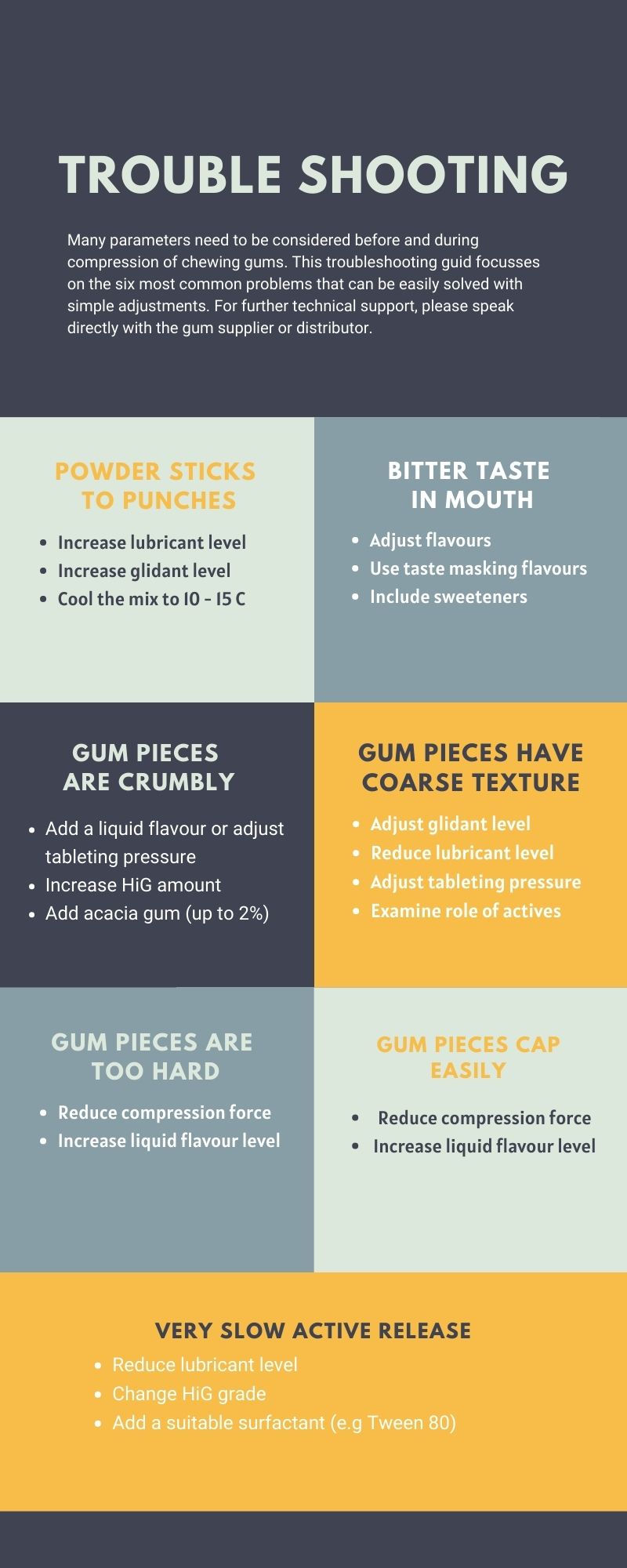
Touble Shooting Guide