Pharmaceutical Flavour | Uses, Suppliers, and Specifications
Flavourings denote one or blends of approved and well-defined chemical substances and extracts with complex olfactory and gustatory properties for use or intended to be used in oral pharmaceutical products as flavourings. Typically, they add a desired taste and odour profile and may enhance, shift, or even mask the intrinsic flavour of the pharmaceutical product.
In the EU, Regulation (EC) No. 1334/2008 on flavourings uses the following terminologies:
Flavouring substance – which is a defined chemical substance with flavouring properties, for example, menthol or ethyl butyrate
Natural flavouring substance – which are flavouring substances naturally identified in nature and obtained by appropriate physical, enzymatic or microbiological steps from material of plant or animal origin, and either in raw state of processed.
Flavouring preparation – which are products other than flavouring substances obtained by appropriate physical, enzymatic or microbiological processes either in raw state or processing. For example, mint extract, vanilla extract or orange oil.
Natural X flavouring – used for flavourings in which the flavouring components exclusively contain natural flavouring substances. For example, Natural strawberry flavour, Natural fruit flavour, etc.
Flavour source material – refers to material of vegetable, animal, microbiological or mineral origin from which flavourings are produced. Examples include fruits, rose petals, spices, etc.
Artificial flavours are any flavours that are not defined as natural, even if they have the exact same chemical composition as flavours isolated directly from nature. This distinction between the origins of flavours has no bearing on how safe, healthy, or delicious they are.
Ethyl decadienoate
| Chemical Structure | 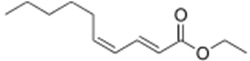 |
| Chemical Formula | C12H20O2 |
| IUPAC name | Ethyl (2E,4Z)-2,4-decadienoate |
| Description | Pear flavour and odour. Found naturally in apples, grapes and pears |
Ethyl maltol
| Chemical Structure | 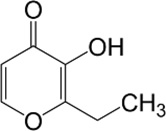 |
| Chemical Formula | C7H8O3 |
| IUPAC name | 2-Ethyl-3-hydroxy-4-pyranone |
| Description | Caramelized sugar, cooked fruit, and cotton candy flavors; this is a synthetic chemical |
Ethyl propionate
| Chemical Structure | 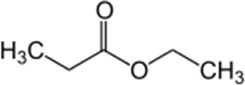 |
| Chemical Formula | C5H10O2 |
| IUPAC name | Ethyl propanoate |
| Description | Fruity flavor, pineapple odor; traces found in strawberries and kiwis. Produced synthetically |
Ethylvanillin
| Chemical Structure | 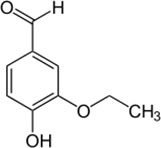 |
| Chemical Formula | C9H10O3 |
| IUPAC name | 3-ethoxy-4-hydroxybenzaldehyde |
| Description | Strong vanilla flavor used in chocolate; it was one of the first (late 19th century) synthetic flavors added to foods |
Eucalyptol
| Chemical Structure | 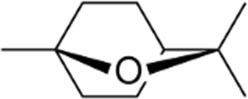 |
| Chemical Formula | C10H18O |
| IUPAC name | 1,3,3-Trimethyl-2-oxabicyclo[2,2,2]octane |
| Description | Eucalyptus – minty smell, spicy, cooling taste, added to mouthwashes and cough medicine (it is a cough suppressant). Produced from eucalyptus leaves |
Isoamyl acetate
| Chemical Structure | 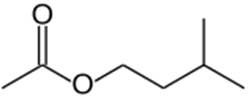 |
| Chemical Formula | C7H14O2 |
| IUPAC name | 3-Methylbutyl acetate |
| Description | Banana and pear flavors; found naturally in bananas, peppermint leaves, and is a fermentation product in beer. Produced synthetically for the food industry |
2,6-Lutidine
| Chemical Structure | 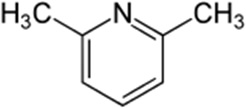 |
| Chemical Formula | C7H9N |
| IUPAC name | 2,6-Dimethylpyridine |
| Description | Nut, bread, coffee aromas when used at low concentrations; found naturally in bread, tea, coffee, cooked beef and pork, cheeses, and beer. Produced synthetically for the food industry |
Limonene
| Chemical Structure | 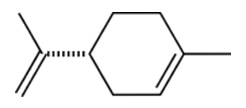 |
| Chemical Formula | C10H16 |
| IUPAC name | 1-Methyl-4-(1-methylethenyl)-cyclohexene |
| Description | Orange and citrus flavors; found naturally in citrus fruit rinds, from which it is extracted for the food industry |
Manzanate
| Chemical Structure | 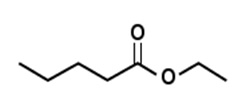 |
| Chemical Formula | C8H16O2 |
| IUPAC name | Ethyl 2-methylpentanoate |
| Description | Apple with cider and pineapple hints; produced synthetically |
Menthone
| Chemical Structure | 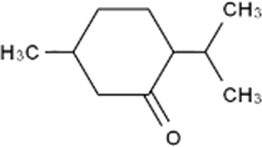 |
| Chemical Formula | C10H18O |
| IUPAC name | (2S,5R)-2-Isopropyl-5-methylcyclohexanone |
| Description | Peppermint odour; small amounts are found naturally in essential oils such as peppermint, and pennyroyal. Produced synthetically for the food industry |
Methyl anthranilate
| Chemical Structure | 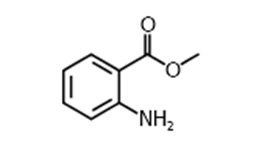 |
| Chemical Formula | C8H9NO2 |
| IUPAC name | Methyl 2-aminobenzoate |
| Description | Grape flavour in Kool-Aid, candy, and chewing gum; found naturally in a number of sources including grapes, oranges, lemons, strawberries, and bergamot. Produced synthetically for the food and perfume industries |
Physicochemical Properties
Powdered Flavours
| Appearance | Solid, powder |
| Colour | Cream, white, characteristic |
| Odour | Characteristic |
| Taste | Characteristc |
| Extractable oil | ≤ 10% |
| Particle size 0.85mm | ≤ 99.0% passes |
| Water content | 0.0 – 10.0% |
| Flash Point | >100 oC |
| Aerobic Plate Count (CFU) | Max 1000/g |
| Coliforms/Enterobacteriacea (CFU) | Max 10/g |
| Mould (CFU) | Max 100/g |
| Yeast (CFU) | Max 100/g |
| Salmonella | Absence |
| E.Coli | Absent |
Liquid Flavours
| Appearance | Fluid, Limpid Liquid |
| Colour | Colourless to yellow, other |
| Odour | Characteristic |
| Taste | Characteristc |
| Extractable oil | Varies |
| Water content | 0.0 – 10.0% |
| Flash Point | <150 oC |
| Specific gravity | 0.8 – 1.2 |
| Refractive Index | 1.2 – 1.6 |
| Aerobic Plate Count (CFU) | Max 1000/g |
| Coliforms/Enterobacteriacea (CFU) | Max 10/g |
| Mould (CFU) | Max 100/g |
| Yeast (CFU) | Max 100/g |
| Salmonella | Absente |
| E.Coli | Absent |
Applications in Pharmaceutical Formulation
Pharmaceutical flavours are available in liquid or powder form, in a variety of concentrations, formulations and sizes.
Pharmaceutical flavours are added to pharmaceutical products for various reasons, but mainly
- to impart flavour to an otherwise bland product. The flavouring may be in imitation of an existing natural flavour or may be created to give some desirable flavour experience
- to impose a different flavour character from that arising from the basic ingredients
- to boost weak intrinsic flavours or replace flavour notes lost during processing
- to modify or complement an existing flavour profile
- to disguise or cover undesirable flavour attributes
- to overcome seasonal variability ‘in natural flavouring materials or constituents
- to impart a flavour where the use of a natural flavouring material is technologically impracticable
One of the major functions of intentionally added flavourings is to extend the range and flexibility of products and processing technology, but their specific application is determined by factors which are not exclusively technical in character. These include:
- Acceptability to the consumer-the product’s flavour is open to wide hedonic interpretation. Preferences display a broad spectrum depending on such factors as ethnic origins, education and upbringing, age, environment, and even one’s personal mood at the time. The strength and quality of flavours are often regionalised. This poses a big problem to the manufacturer aiming at international distribution.
- Regulatory acceptability-this is of increasing concern as the use of flavourings has become heavily regulated in recent years in order to safeguard the consumer from real or supposed health hazards arising from the ingestion of materials intentionally added to the natural diet. A secondary aim is the prevention of deception as to the true nature of the products which the consumer must take on trust. It is essential that any product complies with the legislation of the country in which it is offered for sale.
- The nature of the product and its subsequent preparation for consumption-today the range of consumable products is enormous resulting from rapid advances in processing technology, imaginative product conception, versatile packaging and efficient distribution. The form of the product will determine the form in which flavourings may be incorporated. It is sufficient here to say that dry products call for powdered flavourings and wet products enable one to use flavourings in liquid form.
In deciding the most appropriate flavouring, the product development team must be able to produce a facsimile of the end product under laboratory or pilot plant conditions closely similar to those encountered in full scale production. This is the only sure way of establishing the technological and aesthetic acceptability of flavouring in the finished product.
Evaluation of flavourings in alternative media may be adequate as a first-stage screening but may be quite misleading in relation to the final product.
Many products require further preparation by the consumer. Here, one generally has little or no control. Preparation instructions must be simple and precise but even so, some allowance should be made for indifferent domestic handling. It may be desirable to set flavouring levels a little higher to minimise problems of reducing flavour content to the detriment of product acceptance.
Each of these areas of constraint is complex and makes very special demands on the flavour industry in its service to food processors. Individual flavouring compositions must reflect public tastes and indeed prejudices, as well as catering to unpredictable and often short-lived demands.
Flavourings must be compatible with other prime constituents in the end product, be resistant to processing conditions, and be stable before, during and after incorporation into the finished product.
Very few flavourings are suitable for all applications as processing conditions as well as the physical character of the end product often pose unique problems in flavouring incorporation and subsequent flavour stability. Thus, few generalisations are possible therefore customers should first consider the processing constraints and then to review products by groups in which the processing conditions are broadly similar.
Pharmacopoeial Specifications
Compliance of flavouring constituents with any specific pharmacopoeia is not required.
Safety and Regulatory Status
From a regulatory point of view, pharmaceutical flavours are considered as ‘one excipient’. There are no specific regulations or positive/negative lists on flavourings for pharmaceutical products and by default, when checking flavourings acceptability of flavours, reference is made to food regulations of the relevant country.
It is important to bear in mind that whether flavoured or non-flavoured, registration procedures for pharmaceutical products, both OTC and prescribed, apply, as laid down in Directive 2001/83/EC.
Although both qualitative and quantitative composition of the medicinal product is required, for flavours, it is only necessary to mention the main constituents, with an appropriate method of identification.
For paediatric formulations, the following restrictions apply:
| Excipient | Role in formulation | Potential Adverse effect(s) |
| Propylene glycol | Solvent | Central nervous system (CNS) effects, especially in neonates and children under four years |
| Ethanol | Solvent | Intoxication |
| Polyoxyl castor oil | Vehicle | Severe anaphylactic reactions |
| Polysorbate 80 | Solubilising agent | E-Ferol syndrome, hypersensitivity reactions |
| Benzyl alcohol | Preservative | ‘Gasping syndrome’ in neonates |
| Benzoic acid | Preservative | Jaundice in neonates |
| Parabens (methyl-, ethyl- and propyl-hydroxybenzoates) | Preservative
|
Suggestion of oestrogenic activity with potential reproductive effects (with propylparaben), hypersensitivity reactions, hyperbilirubinaemia in neonates |
| Benzalkonium chloride | Preservative | Bronchospasm from anti-asthmatic drugs |
| Sodium metasulfites | Antioxidant | Wheezing, dyspnoea and chest tightness in asthmatic children |
| Sorbitol | Sweetener | Osmotic diarrhoea and gastrointestinal discomfort |
| Mannitol | Sweetener | Osmotic diarrhoea and gastrointestinal discomfort |
| Glucose and sucrose | Sweetener | Obesity and tooth decay |
| Saccharin | Artificial sweetener | Hypersensitivity and photosensitivity reactions |
| Aspartame | Artifical sweetener | A source of phenylalanine that should be avoided in patients with phenylketonuria |
| Colourants | Colouring agents | Sensitivity reactions and hyperactive behaviour in children[ |
Note that flavourings are considered as ‘single’ excipients from a regulatory point of view even though they consist of several ingredients. However, flavouring may well contain components which in their own right are considered as excipients and therefore require labelling.
Stability and Storage Conditions
Liquid flavours are volatile and preparations may exhibit thermodynamic instability. The prolonged action of heat may induce physical separation into separate phases. Most liquid flavours are stable for at least 12 months if stored in unopened airtight containers at room temperature (<25 0C).
The pH may drop slightly during storage, which should be taken into account. Separation into phases may also occur, but agitating the hot solution can reverse this.
Flavour powder should be stored in an airtight, corrosion resistant container and protected from moisture. The use of glass, plastic, or resin-lined containers is recommended for the storage of formulations containing carbomer.
Handling Precautions
Observe normal precautions appropriate to the circumstances and quantity of material handled. Eye protection and gloves are recommended.
References

