Excipients and Formulation Approaches Employed in Leading Covid-19 Vaccines
In this article, I compare excipients and formulation methods used in the four Covid-19 vaccines from Pfizer BioNTech, Moderna, Astra Zeneca and Janssen-Cilag (Johnson & Johnson), that have obtained emergency approval by the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) and its European counterpart, the European Medicines Agency, EMA.
Introduction
SARS Covid-19 has put vaccines into the public limelight again. Now more than before, we are all aware of the need for and challenges of timely distribution, as well as the importance of the cold chain. What is perhaps not clear to most people is that these challenges are heavily influenced by a vaccine’s inactive ingredients (excipients, solvents & adjuvants) as well as the platform.
In addition to stability, a vaccine’s formulation and excipients impact the economics of manufacture and the finished product’s presentation. It is the same way an engineer at Ferrari or Aston Martin designs an impressive V8 powertrain but finds that he or she still needs an equally impressive chassis for the new engine to deliver the performance.
This is why an understanding of how vaccines are formulated and the reasons behind choice of different excipients, from a pharmaceutical technology perspective, is equally important to appreciating differences in manufacturing, storage and distribution requirements.
First, I will briefly provide an outline of the vaccine platforms currently in use as this is important to formulation and excipients selection.
Vaccine Platforms
There are any number of ways to categorise vaccines. One method I like to use is classify them by the technology or platform used. Using this approach, we can distinguish four main platforms:
Whole pathogen vaccines
This is the oldest and most well-known method of vaccine development. It involves using an entire disease-causing organism in the vaccine to elicit the immune response, analogous to that obtained in regular infection. Whole pathogen vaccines are further divided into live attenuated and inactivated vaccines.
In live attenuated vaccines the disease-causing organism is weakened (attenuated) to curtail its disease-causing ability although it’s still able to replicate and trigger an immune response. An example is the Oral Polio Vaccine.
Inactivated vaccines have the genetic material destroyed – this way they are not able to replicate and infect cells, but are still able to trigger an immune response. Since inactivated vaccines do not always create a strong immune response as live attenuated vaccines, adjuvants (for example, aluminium hydroxide and aluminium phosphate are included in the formulation. An example is the Hepatitis A vaccine.
Subunit vaccines
These vaccines typically contain one or more immunogens from the surface of the pathogen. Antigens are usually produced through recombinant technologies. Subunit vaccines can be further divided into recombinant protein vaccines; toxoid vaccines, conjugate vaccines, virus-like particles and outer membrane vaccines.
The vast majority of vaccines in use today are subunit vaccines – they do not contain any whole bacteria or viruses and instead contain polysaccharides or proteins or their combination from the surface of bacteria or viruses, which are recognised by the immune system.
Agencies such as the World Health Organisation and the CDC, attest to the excellent safety profiles of subunit vaccines. Their only ‘downside’ is that they often require inclusion of adjuvants. Examples of subunit vaccines include
Nucleic acid vaccines
Nucleic acid vaccines work by providing genetic codes for host cells to produce antigens, which then stimulate the immune response. Nucleic acid vaccines can be further divided into RNA and DNA vaccines.
RNA vaccines use mRNA which is formulated in a lipid nanoparticle for protection and fusion with the cell membrane. A drawback of RNA vaccines is their inherent instability.
DNA, being more stable than mRNA, doesn’t require the same initial protection. DNA vaccines are typically administered using electroporation to allow cells to take up the DNA. There are currently no licenced DNA vaccines, however there are several in different stages of development.
Viral vectored vaccines
Viral vectored vaccines utilise harmless viruses to deliver the genetic code of target vaccine antigens to cells of the body, so that they can produce protein antigens to stimulate an immune response. Viral vectored vaccines can be developed quickly and on a large scale. They are also significantly cheaper to produce compared to nucleic acid or subunit vaccines.
Viral vectored vaccines can be further classified into replicating and non-replicating. In the former, viral vectors retain the ability to make new viral particles alongside delivering the vaccine antigen when used as a vaccine delivery platform. Non-replicating, as the name suggests, do not retain the ability to make new viral particles because some of the viral genes required for viral replication have been removed.
Differences by Vaccine Platform
The Pfizer BioNtech and Moderna vaccines are nucleic acid vaccines. Both the Pfizer BioNTech Covid-19 vaccine (BNT162b COVID-19 mRNA vaccine) and the Moderna Covid-19 vaccine (mRNA-12743 COVID-19 vaccine) are single stranded, 5’ capped messenger RNA produced by cell-free in vitro transcription from corresponding DNA templates that encode for SARS-Cov-2 spike protein.
Although mRNA vaccines are a relatively new technology (approx. 30 years old, compared to whole organism vaccines that were first introduced during the late 1700s), they are well studied. They also offer many advantages:
- Firstly, no live components are involved, so there is no risk of the vaccine triggering disease.
- The mRNA, due to its transient nature, also presents zero risk of becoming integrated with our own genetic material.
- Moreover, the immune response involves both B and T cells.
- Finally, and perhaps more importantly, they are relatively easy to manufacture.
The major downsides of mRNA vaccines are that they often require ultra-cold storage, and almost always require booster shots for maximum effectiveness. We will touch on this later in the article.
By comparison, the Jansen-Cilag and AstraZeneca vaccines are viral vectored vaccines, so they use a different approach to instruct human cells to make the SARS-2 spike protein. The Jansen-Cilag Covid-19 vaccine (COVID-19 vaccine (Ad26,COV2-S [recombinant])) uses a non-replicating adenovirus (a Novel Adenovirus Type 26) from their AdVac technology and grown in PER.C6 cell line.
The AstraZeneca Covid-19 vaccine (COVID-19 (ChAdOx1-S [recombinant]) also uses a non-replicating adenovirus, this one being a chimpanzee adenovirus (as opposed to Ad26, which is human adenovirus) known as Oxford1 (or ChAdOx1).
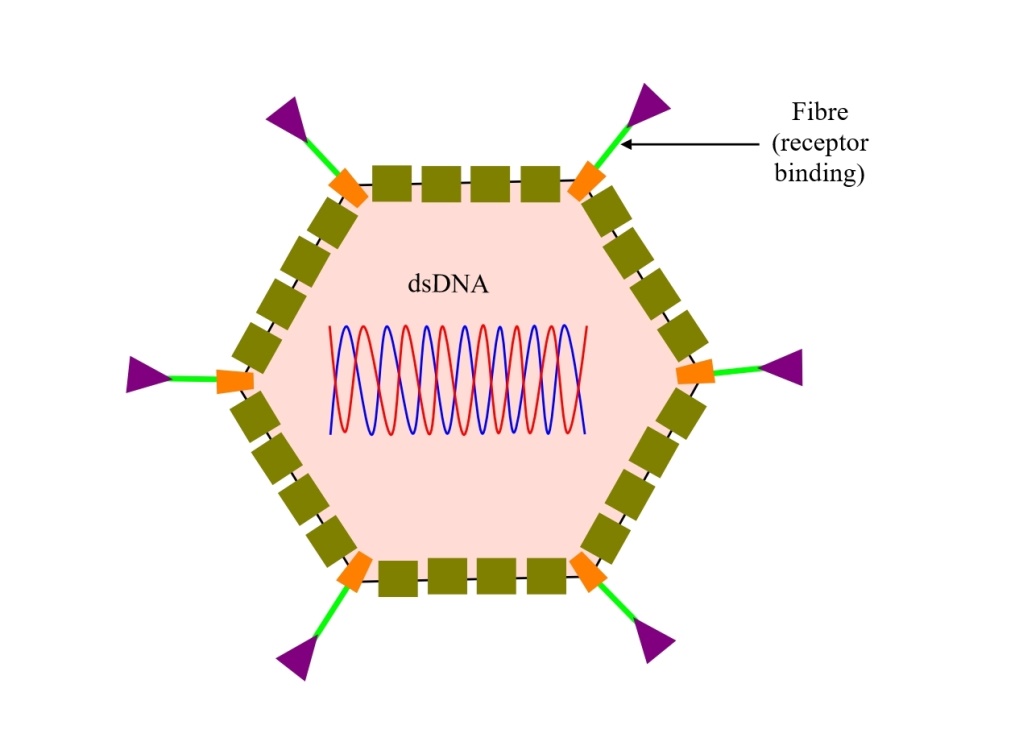
A schematic illustration of an adenovirus vector vaccine is shown below:
Fig. 1: Schematic illustration of an adenovirus vector vaccine
The adenoviruses used in these vaccines are engineered to only carry the genetic code for the SARS-2 spike protein. Upon entering human cells, they use that code to make spike proteins. These vaccines mimic natural infections, which is advantageous in triggering strong cellular immune responses as well the production of antibodies by B cells.
The technology is well-established, with two other vaccines already approved (Ebola & Zika vaccines). However, adenovirus vaccines are relatively complex to manufacture, and with time, their effectiveness reduces.
How Covid-19 Vaccines Differ in Formulation and Excipients Used
Pfizer BioNtech and Moderna Vaccines
The Pfizer BioNtech and Moderna vaccines are available as sterile, multi-dose colloidal dispersions for intramuscular injection. The mRNA in both vaccines is encapsulated in lipid nanoparticles (LNPs). LNPs are chosen to overcome the inherent hydrolytic instability, poor membrane permeability, and the abundance of RNAses in the body.
Lipid nanoparticles (sometimes called solid lipid nanoparticles, SLNs) are colloidal carriers made from lipids. As a drug delivery technology, LNPs emerged in the early 1990s as an alternative to traditional emulsions and liposomes. Although their exact structure is still under debate, LNPs are generally thought to consist of a solid lipid core (unlike liposomes which have an aqueous core) and an external phospholipid layer (membrane). A schematic illustration of an LNP is shown in figure 2. You can also watch an excellent YouTube on LNPs through this link.
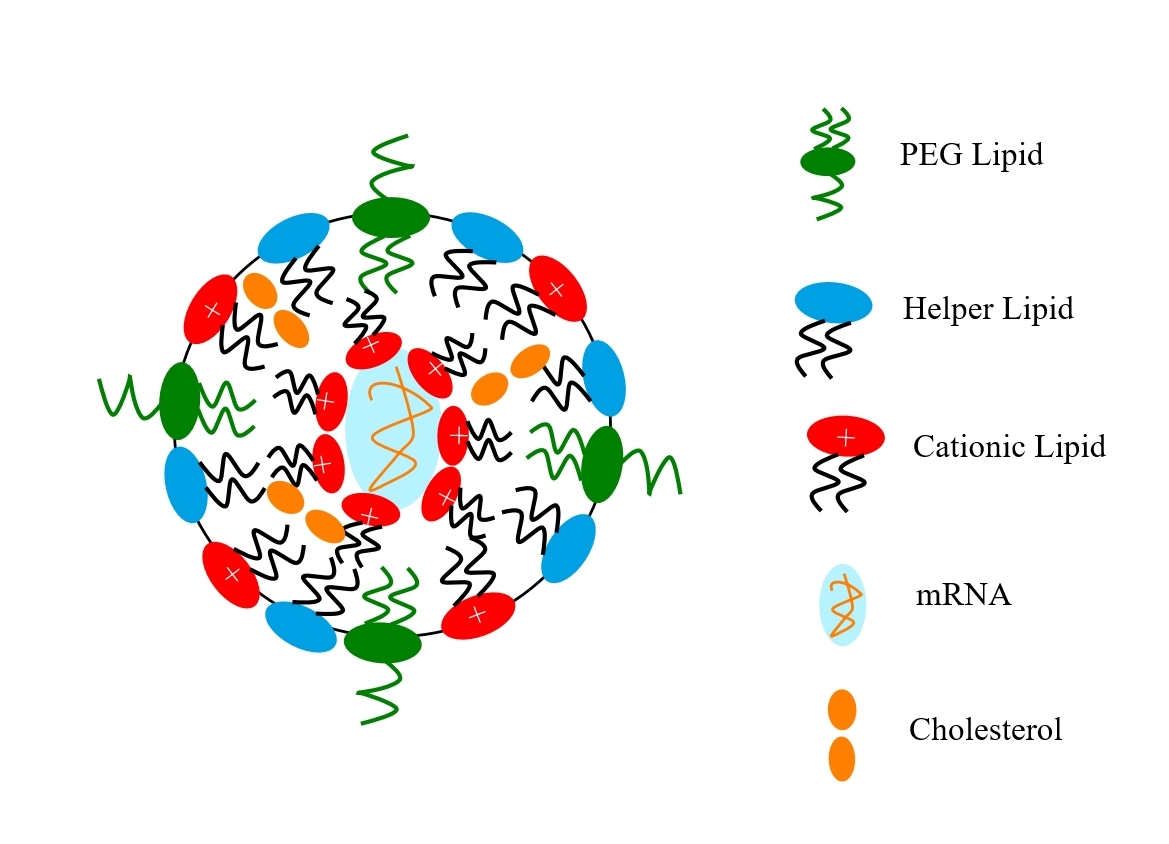 Fig. 2: Schematic illustration of an lipid nanoparticle vaccine
Fig. 2: Schematic illustration of an lipid nanoparticle vaccine
For the Pfizer BioNtech and Moderna vaccines, LNPs are obtained by admixing mRNA, various lipids, which include a neutral phospholipid, cholesterol, a polyethylene-glycol (PEG)-lipid, and an ionizable cationic lipid (which has amine groups (at low pH) and facilitates interaction with the anionic mRNA during particle formation and also membrane fusion during internalization). The PEG-lipid controls particle size and acts as a steric barrier, preventing aggregation during storage. When complexed with the mRNA, the LNPs-mRNA particles have sizes in the range of 60–100 nm.
Table 1 below summarises the main differences in Pfizer BioNtech and Moderna vaccines’ formulation and excipients:
| Pfizer-BioNTech vaccine | Moderna vaccine | |
| Name of product | Comirnaty | mRNA-1273 |
| Active | BNT162b2 (single-stranded, 5’ capped mRNA) | mRNA 1273 (single-stranded, 5’ capped mRNA) |
| mRNA dose; route of administration | 30 µg; intramuscular | 100 µg; intramuscular |
| Delivery system | Lipid nanoparticle made from
ALC-0315 or 4-hydroxybutyl) azanediyl)bis (hexane-6,1-diyl)bis(2-hexyldecanoate), ALC-0159 or 2-[(polyethylene glycol)-2000]-N,N ditetradecylacetamide; 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) and Cholesterol |
Lipid nanoparticles made from
SM-102 (heptadecan-9-yl 8-((2-hydroxyethyl) (6-oxo-6-(undecyloxy) hexyl) amino) octanoate}; PEG2000-DMG = 1-monomethoxypolyethyleneglycol-2,3-dimyristylglycerol with polyethylene glycol of average molecular weight 2000; 1,2-Distearoyl-sn-glycero-3 phosphocholine (DSPC) and Cholesterol |
| Diluent | Water for injection
Buffered by Potassium dihydrogen phosphate, Disodium hydrogen phosphate dihydrate pH 7–8 |
Water for injection
Buffered by Tris (tromethamine) pH 7–8 |
| Other excipients | Potassium chloride
Sodium chloride Sucrose |
Sodium acetate
Sucrose |
Table 1: Formulation of Pfizer BioNtech and Moderna COVID-19 vaccines
LNPs are particularly unstable thermodynamically. In addition, they are susceptible to chemical instability, which can arise from hydrolysis and oxidation of the lipids in the LNPs, as well as oxidation of unsaturated fatty acid groups. This makes LNPs systems especially susceptible to storage conditions, which helps explain, in part, to the stringent handling conditions required of mRNA vaccines.
Janssen-Cilag (Ad26. COV2.S) and AstraZeneca (Vaxzevria or AZD1222)
Janssen-Cilag (Ad26. COV2.S) and AstraZeneca (Vaxzevria) vaccines are available as sterile, multi-dose aqueous suspensions for intramuscular administration. Liquid suspensions are an efficient and the go-to format for viral vector gene delivery systems. However, the challenge faced by formulators is ensuring long term stability since, unlike conventional pharmaceutical products, they are complex biological structures susceptible to chemical and physical stressors, such as changes in solution pH, ionic strength, redox potential and surface activity.
Thus, the aim of formulation efforts here is to prevent conditions likely to trigger degradation pathways, such as denaturation of the capsid protein and nucleotides, aggregation, hydrolysis and precipitation and adsorption of the vaccine onto the container walls. This mandates the use of buffers as well as functional excipients and other materials in the formulation, such tonicity agents and stabilisers, non-ionic surfactants to prevent adsorption to glass surfaces and cryoprotectants (sucrose, ethanol or cyclodextrins), free-radical oxidation inhibitors and metal chelators (edetate).
Table 2 below summarises the main differences between Janssen-Cilag and AstraZeneca Covid-19 vaccines’ formulation and excipients:
| AstraZeneca vaccine | Jansen-Cilag vaccine | |
| Name product | Vaxzevria (formerly AZD1222) | COVID-19 Vaccine Janssen |
| Active | ChAdOx1-S [Recombinant] | Ad26. Cov2.S [Recombinant] |
| Dose & route of administration | 0.5 ml; intramuscular
(containing ³ 2.5×108 Inf. Units) |
0.5ml ml; intramuscular
(containing ³ 8.3×108 Inf. Units) |
| Delivery system | Replication-deficient, non-encapsulated Chimpanzee adenovirus ChAdx1-S encoding SARS-COV-2 spike (S) glycoprotein
Each virion is 80-100nm and contains a single copy of double-stranded DNA |
Replication-deficient, non-encapsulated adenovirus type 26 (Ad26) encoding SARS-COV-2 spike (S) glycoprotein
Each virion is 80-100nm and contains a single copy of double-stranded DNA |
| Diluent | Water for injections | Water for injections |
| Buffer system | L-histidine, L-histidine hydrocholoride monohydrate
pH = 6.6 |
Citric acid monohydrate, Trisodium citrate dihydrate
pH = 6.0-6.4 |
| Other excipients | Magnesium chloride hexahydrate
Ethanol Sucrose Sodium chloride EDTA |
HCl
2-hydroxyl propyl β-cyclodextrin Ethanol Sodium chloride Sodium hydroxide |
Table 2. Formulation and Excipients used in Janssen-Cilag (Ad26. COV2.S) and AstraZeneca (AZD1222) COVID-19 vaccines
Differences in Storage Requirements
All vaccines (with the exception of a select few) require high quality and robust cold chains to guarantee stability and viability. These conditions are not arbitrary – they are arrived at from extensive stability studies and conditions where the viability of the products is monitored.
As hinted to previously, the mRNA vaccines are especially vulnerable to handling conditions, hence their requirements are particularly elaborate compared with adenovirus vaccines.
Of the two mRNA vaccines, Pfizer’s is the more challenging to handle, requiring shipping and storage in ultra-cold freezers. I was not able to find any studies on storage stability in the public domain on mRNA COVID-19 vaccines, however Onpattro® , a marketed LNP product has a shelf-life of 36 months when stored between 2° and 8 °C. It is possible that in future, these conditions will be updated as more storage stability data emerge.
A summary of the key requirements for the different vaccines is below:
Pfizer BioNTech COVID-19 Vaccine
- 6 months maximum shelf life when stored in a freezer at -80°C to -60°C
- 31 days maximum shelf life at 2-8°C after thaw
- May be stored between 2 to 25°C for 2 hours prior to dilution after removal from the fridge
- Once diluted may be stored between 2 to 25°C for a further 6 hours
- Protect from room light and direct sunlight or UV light
Moderna COVID-19 Vaccine
- 7 months maximum shelf life when stored in a freezer at -25°C to -15°C
- 30 days maximum shelf life at 2 to 8°C after thaw
- May be stored between 8 to 25°C for up 12 hours prior to dilution after removal from the fridge
- Once punctured, the vial must be used within 6 hours
- Protect from room light and direct sunlight or UV light
AstraZeneca COVID-19 Vaccine
- 6 months maximum shelf life when is stored in a refrigerator between 2 to 8°C
- 6 hours maximum shelf life when stored between 2 to 25°C.
- Once punctured, the vial must be used within 6 hours
- Must not be frozen
- Protect from room light and direct sunlight or UV light
Vaccine Janssen-Cilag COVID-19
- 24 months maximum shelf life when is stored in a freezer between -25 and -15 °C
- 3 months maximum shelf life when stored between 2 to 8 °C after removal from freezer.
- Once punctured, the vial must be used within 6 hours
- Must not be frozen
- Protect from room light and direct sunlight or UV light
So there you have it. A summary of platforms for Covid-19 vaccines, the formulations and excipients used, and how these influence handling, storage and distribution requirements of Pfizer BioNTech, Moderna, Astra Zeneca and Janssen-Cilag (Johnson & Johnson)’s vaccines.
References
- Schoenmaker, D. Witzigmann, J.A. Kulkarni, R. Verbeke, G. Kersten, W. Jiskoot, D.J.A. Crommelin, mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability, International Journal of Pharmaceutics, 601 (2021) 120586.
- S. Rosa, D.M.F. Prazeres, A.M. Azevedo, M.P.C. Marques, mRNA vaccines manufacturing: Challenges and bottlenecks, Vaccine, 39 (2021) 2190-2200.
- D’Amico, F. Fontana, R. Cheng, H.A. Santos, Development of vaccine formulations: past, present, and future, Drug Delivery and Translational Research, 11 (2021) 353-372.
- Mäder, K. Solid lipid nanoparticles, Handbook of Materials for Nanomedicine, Jenny Stanford Publishing 2020, pp. 173-206.
- Tatsis, N., Ertl, H.C., Adenoviruses as vaccine vectors, Molecular Therapy, 10 (2004) 616-629.