Dr. Enosh Mwesigwa, Pharmacentral Guest Columnist
This article reviews several antimicrobial actives widely used for antisepsis and disinfection. The focus is on the performance attributes of the most important broad-spectrum biocides and their use in homes and institutional settings.
Introduction
It is a plain fact that our ability to effectively control microbial proliferation is an innovation of the same standing as electricity and the internet in facilitating our modern ways of living.
Biocides supplement chemotherapeutics and underpin management of pathogen proliferation in food production, healthcare settings and many other environments where critical activities take place. Put simply, without biocides life would not be very different.
But first, what is a biocide? Generally the term biocide describes chemical agents that inactivate microorganisms (bacteria, fungi and protozoa), as well as viruses. Owing to its general applicability, other more specific terms, such as bactericide or fungicide, etc are often used. For more information on terminologies used, click here.
There are currently many different biocides available for use in antiseptic and disinfectant products, many of these have been in use for several years. They require careful selection since their indiscriminate and/or incorrect application can lead to sub-optimal results, and in the worst case scenarios, the development of resistant strains with catastrophic consequences for human welfare.
The purpose of this article is to review the properties and advantages of a selection of broad spectrum biocides with respect to their use in antisepsis, preservation and/or disinfection in the home or institutional settings.
Criteria used to select biocide
Antimicrobial activity is affected by many different factors, including formulation, presence of organic load, synergies, temperature, and concentration. With the emergence of resistant organisms the importance of using the most appropriate biocide products has become especially important.
Thankfully, there are guidelines from health protection agencies, such as the World Health Organisation and the Centre for Disease Control that we can use when evaluating a biocide product or chemistry:
Speed of action
Antimicrobial agents do not exert their actions immediately upon contact with microorganisms. Each product has a minimum “contact time” that’s needed in order for the biocide to exert antimicrobial effects.
In the case of disinfectants, contact time must be adhered to in order to achieve terminal disinfection. For instance, if a product were to dry out from the surface before its contact time has elapsed complete disinfection may not be achieved.
The ideal biocide is one that offers rapid and practical contact time, unaffected by formulation excipients, organic load or other external conditions.
Spectrum and Efficacy
In the microbial underworld, no single microorganism has a monopoly over the ability to cause us harm. And unfortunately for us, it is not possible to detect beforehand which microorganism is present on a given surface at a given time.
For this reason, having the option to select broad-spectrum biocides, including action on bacteria, spores, fungi and all viruses is highly beneficial. Thus, the ideal biocide has broad antimicrobial effectiveness, with the ability able to stop the proliferation of a broad range of microorganisms, including resistant strains.
Cleaning Capacity
A biocide’s cleaning properties are often overlooked, with emphasis instead being placed on spectrum or killing speed. However, cleaning efficacy is equally important because dirt and organic matter can create a barrier or protective reservoirs for pathogens.
Thus, a biocide that is also an effective cleaning agent can help eliminate the requirement for a secondary cleaning product. Biocides with concurrent surface active properties or products that include surfactants exhibit enhanced cleaning efficacy while ensuring complete and even coverage of surfaces with the biocide.
Compatibility
The optimum biocide is one that is compatible with and suitable for use on all surfaces. At the moment, there is no single material that exhibits 100 percent compatibility with all surfaces. A number of biocides do show excellent material compatibility profiles for their intended uses, but also fail miserably for particular applications or substrates.
For this reason, understanding and taking into account the likely uses of a biocide when formulating a product is necessary. Failure to do this can predispose users to reduced protection and greater harm.
User Health and Safety
As society modernises and ages the needs for infection control also grow, not just within traditional settings such as hospitals, but in the community and industrial settings as well. The resulting increase in exposure to biocides requires safer chemistries to be available. Thus, when selecting a biocide, chemistries that are non-toxic, non-irritating and non-sensitizing are highly desirable.
Environmental Profile
Increased introduction of biocide chemicals into the environment intensifies the need for chemistries that are sympathetic to the environment. It should not be forgotten that biocides are by their nature killing machines for microbes, both good and bad. Products that are non-selective, especially for aquatic life, are not sustainable in the long-run.
Properties and benefits of broad-spectrum biocides
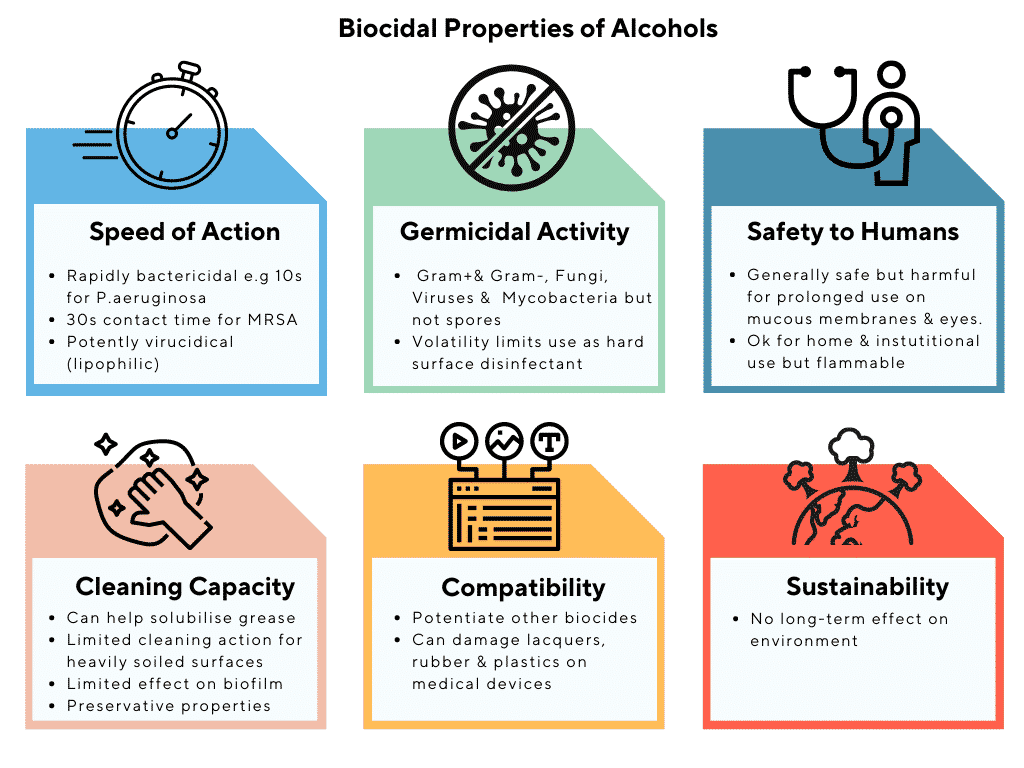
Alcohols
In the context of biocides, ‘alcohols’ refer to a select number of compounds having the OH functional group and exhibiting antimicrobial properties. In this regard, ethyl alcohol, isopropyl alcohol and n-propanol are the most relevant.
The biocidal effect of alcohols is achieved through denaturation of proteins. Alcohols show rapid broad-spectrum activity against vegetative bacteria, mycobacteria, viruses and fungi. They inhibit sporulation but they are not sporicidal. Isopropyl alcohol is more effective against bacteria while ethyl alcohol is more potent against viruses.
Usage and Applications
Alcohols are mainly used as skin antiseptics in a variety of consumer products. Antimicrobial activity is optimal in the 60 to 90% concentration range. Lower concentrations can be used as preservatives and to potentiate other biocides e.g chlorhexidine.
However, due to their lack of sporicidal effects and inability to penetrate protein-rich materials, alcohols are not used as a sterilants for medical and surgical devices except for small implements, such as thermometers, pagers, scissors and stethoscopes but not for hard surfaces.
Alcohol wipes are used to disinfect small surfaces such as rubber stoppers of multi-dose vials or vaccine bottles or external surfaces of equipment.
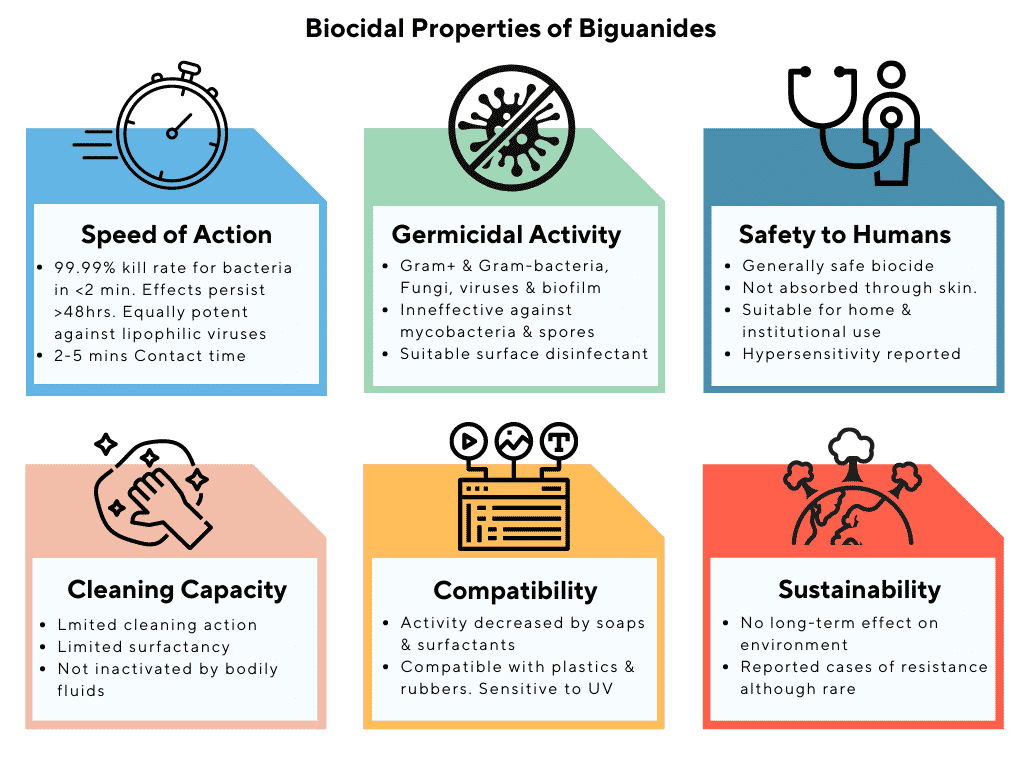
Biguanides
Biguanides are organic compounds with the biguanide [-HN(C(NH)NH2)2-] functional group. Chlorhexidine is the most widely used biguanide within the biopharmaceutical field but other biguanides are also available, including polyhexanide and alexidine.
 Chlorhexidine
Chlorhexidine
Chlorhexidine is a cationic bisbiguanide that has been used in the UK over several decades. Depending on concentration, it exhibits both bacteriostatic and bactericidal action, mediated via its ability to disrupt microbial cell membranes and precipitation of cell contents.
Chlorhexidine is known for being highly effective against Gram-positive organisms as well as against Gram-negative bacteria (at high concentrations). It is effective against fungi, yeasts and enveloped viruses, such as SARS COV 2 and HIV. Chlorhexidine has a much quicker kill rate than many other antimicrobial agents. Activity against mycobacteria and spores is, however, limited.
 Usage and Applications
Usage and Applications
Chlorhexidine is used in consumer topical products as an antiseptic. Typical usage levels are 2-4% in topical products.
It is also used as catheter lubricant (intra-urethral), as well as a component of medicated mouthwashes (0.12%). A number of medical devices, such as implanted surgical mesh, intravenous catheters and topical dressings incorporate chlorhexidine.
Within hospitals chlorhexidine is used as a disinfectant to prevent spread of antimicrobial-resistant organisms. In this respect, it is often referred to as the gold standard for infection reduction.
Iodine and iodophors
Iodine’s biocidal effects have long been known. However, owing to its skin staining and irritating effects, iodine is largely superseded by iodophors as the active ingredient in these antiseptics.
Iodophors consist of elemental iodine, iodide or triiodide, bound to a polymer carrier (complexing agent) of high molecular weight, such as povidone or poloxamer. Complexation of iodine with a polymer increases the solubility of iodine, promotes sustained-release of iodine and reduces skin irritation.
The biocidal mechanism of action is via the formation of complexes with amino acids and unsaturated fatty acids in the cystol of microorganisms, impairing protein synthesis.
 Usage and Applications
Usage and Applications
Iodine is potently bactericidal against Gram-positive, Gram-negative and some spore-forming bacteria. It is also active against mycobacteria, viruses and fungi. Povidone-iodine is a safe and effective antiseptic. Most preparations used for hand hygiene contain 7.5–10% povidone-iodine.
An important property of iodophors is their “available Iodine” which refers to the total amount of iodine that can be titrated with sodium thiosulfate. Typical 10% povidone-iodine formulations contain 1% available iodine and yield free iodine concentrations of 1 ppm.
The downsides to iodine compounds is that antimicrobial activity can be affected by pH, temperature, exposure time, concentration of total available iodine and the amount and type of organic and inorganic compounds present (e.g. alcohols and detergents).
Silver Compounds
Silver is a transitional metal element. It may exist as a pure element, as an alloy in combination with other elementals, as a mineral or as an organo-metallic compound. Silver and silver compounds have a long history of use as antimicrobial agents. Some of the well-known derivatives include silver sulfadiazine, silver nitrate and nano or colloidal silver.
Silver and silver compounds have a long history of use as antimicrobial agents. Some of the well-known derivatives include silver sulfadiazine, silver nitrate and nano or colloidal silver. Silver’s antimicrobial action is thought to be through:
- Pore formation and puncturing of the bacterial cell wall when silver ions react with peptidoglycan components
- Entry of silver ions into the bacterial cell, inhibiting respiration and other metabolic pathways, and
- Disruption of DNA replication.
Usage and Applications
Silver has a broad spectrum of microbial activity. It is active against bacteria, fungi and viruses. Activity is more pronounced against Gram negative bacteria than Gram positive bacteria. Colloidal silver nanoparticles are advantageous due to their lower toxicity and higher antimicrobial activity due to their ability to penetrate bacterial cells more easily.
At present, the main use of silver compounds is in the area of topical chemoprophylaxis of burns. In this sense, silver-impregnated dressings and antimicrobial coatings are commercially available for use in infection management and stimulation of wound healing as well as in dental amalgam.
Silver is also impregnated into medical devices, such as heart valves. Silver nitrate and Silver sulfadiazine are used as topical antibacterials for the treatment of skin infections, including acne.
Quaternary Ammonium Compounds
Quaternary ammonium compounds are a group of related compounds composed of a nitrogen atom linked directly to four alkyl groups of varying structural complexity.
In the biopharmaceutical industry, commonly used quaternary ammonium compounds are benzalkonium chloride, cetrimide, cetylpyridium chloride and cetrimonium bromide (cetyltrimethylammonium bromide (CTAB)).
While primarily bacteriostatic and fungistatic, quaternary ammonium compounds can be virucidal, too, particularly at high concentration. Biocidal action is initiated through adsorption of the alkylammonium cation on the cell surface, diffusion through the cell wall and disruption of cytoplasmatic membrane, which releases potassium ions and other constituents, leading to the death of the cell.
Benzalkonium chloride, one of the most common QUAT, is actually a mixture of closely related QUATS (mainly benzyl (dodecyl) dimethyl ammonium chloride, benzyl (tetradecyl) dimethyl ammonium chloride). It is a cationic surfactant with broad-spectrum antimicrobial activity, making it suitable for use as a preservative, antiseptic, disinfectant, solubilising and wetting agent.
Cetrimide mainly consists of trimethyltetradecylammonium bromide and smaller amounts of dodeyl trimethylammonium bromide. It exhibits good bactericidal activity, particularly against Gram positive species but is less so active against Gram negative species. Pseudomonas aeruginosa, may exhibit resistance. Antifungal activity is variable and it is inactive against bacterial spores and viruses.
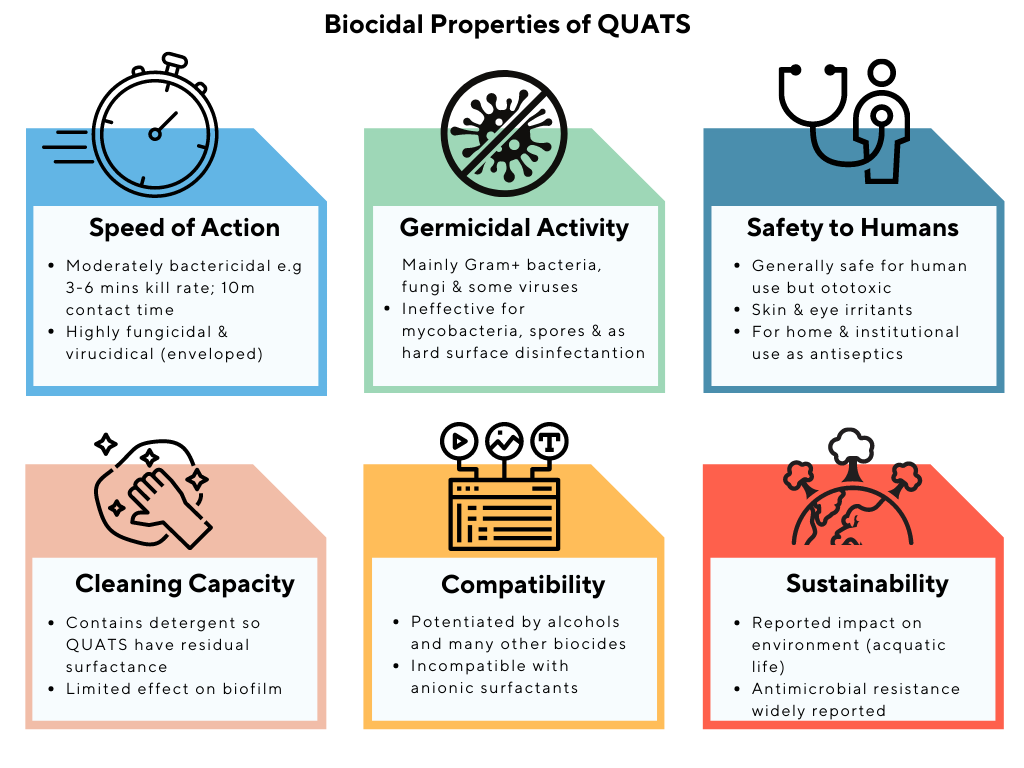 Usage and Applications
Usage and Applications
Benzalkonium chloride is widely used in cosmetics, wet wipes, hand and surface sanitisers. It has also been used as a spermicide.
Benzalkonium chloride has also been formulated into lozenges for use in the treatment of common mouth and throat infections. It remains one of the most commonly used preservatives in ophthalmic and nasal preparations.
Cetrimide, on the other hand, functions as an antimicrobial preservative in cosmetics and pharmaceutical eye drops and topical formulations as well as an antiseptic in topical creams, sprays and medicated shampoos.
The other uses are as a cationic surfactant, cleanser and disinfectant for hard contact lenses.
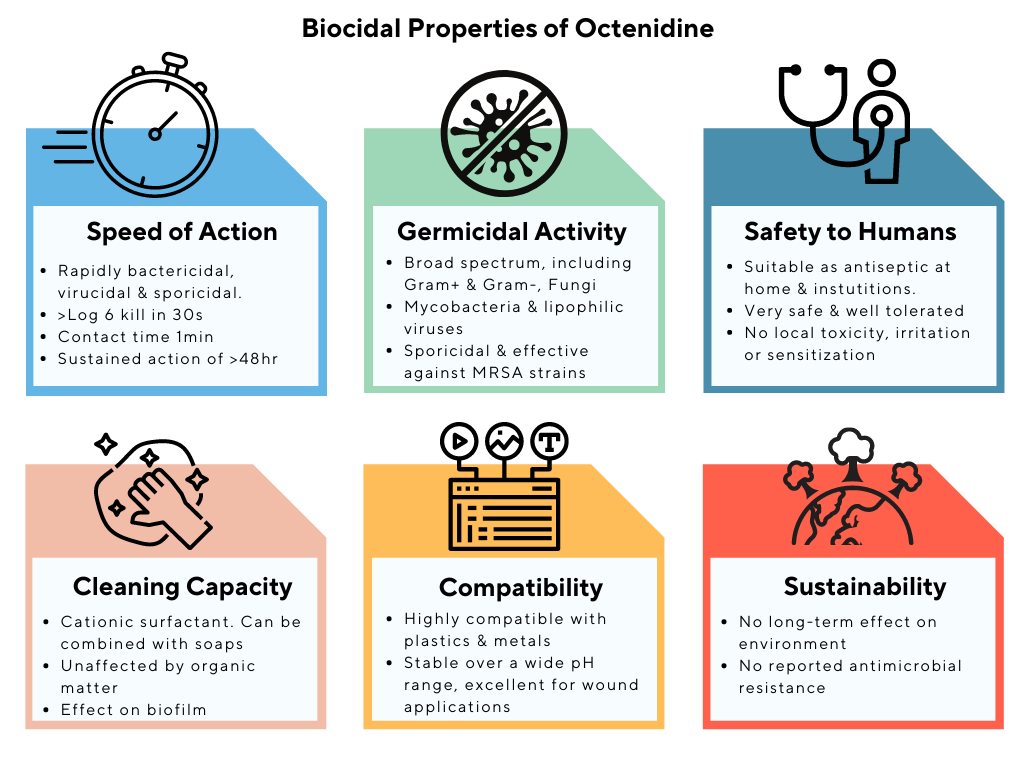
Bispyridines
Octenidine dihydrochloride is a cationic antiseptic that belongs to the bispyridine class of chemical substances. A relatively new biocide, Octenidine was developed just over two decades ago. It exhibits broad antimicrobial activity and is effective against biofilm-forming organisms, including MRSA, plaque-forming bacteria such as Actinomyces and Streptococcus, Chlamydia and Mycoplasma and also binds to negatively charged surfaces.
Usage and Applications
Octenidine dihydrochloride is a versatile biocide intended for use in human and all animal species for skin and mucosal disinfection. It works well as short-term supportive antiseptic in wound treatment as a 0.1% aqueous solution for cutaneous use as well as for long-term antisepsis.
In addition, octenidine is used for sterilization of IV catheters, and in dental medicine, it is used as mouth rinse, gutta-percha disinfectant, for biofilm inhibition on restorative materials, and as an irrigant in root canal procedures. It has been utilised in mouth rinses to prevent plaque and gingivitis, as well as whole body wash for methicillin-resistant S. aureus decolonization and for skin disinfection of premature new-born infants.
Conclusions
The true test of a biocide is to demonstrate that the product has reduced surface bioburden, prevented proliferation or killed microorganisms. Given the variety of factors at play, the selection of an appropriate biocidal agent can be a complex and challenging process.
Finally, the selection of biocides should not be considered as a one-off decision; it must remain part of the on-going quality review process of the formulator and institutional user.
References
McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999 Jan;12(1):147-79. doi: 10.1128/CMR.12.1.147. Erratum in: Clin Microbiol Rev 2001 Jan;14(1):227. PMID: 9880479; PMCID: PMC88911.
Bloomfield, S. F. Resistance of bacterial spores to chemical agents. In A. D. Russell, W. B. Hugo, and G. A. J. Ayliffe (ed.), Principles and practice of disinfection, preservation and sterilization, 3rd ed., 2013. Blackwell Science, Oxford, England.
CITATION
When referring to this article, please cite as: E. Mwesigwa, A comparative appraisal of the utility of broad-spectrum biocides. Pharmacentral Science and Technology Bulletin 01 (09) 2021
post1