| Weight | 5 kg |
|---|
Saccharin Excipient | Uses, Suppliers, and Specifications
Saccharin is a synthetic, white, crystalline powder, that contains the -CONHSO2– (N-sulfonyl amide) structural unit, common to several compounds with a sweet taste. In its pure state, saccharin is 550 times sweeter than sugar. it was discovered in 1879 by Constantin Fahlberg and the first widely commercialized non-nutritive sweetener.
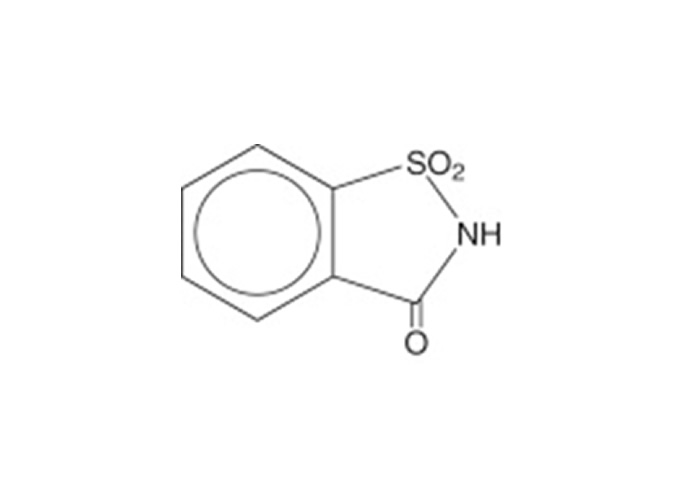
The general chemical structure of Saccharin is shown in Figure 1:
Figure 1: Chemical Structure of Saccharin
| Chemical Name | 1,2 Benzisothiazol -3(2H) – one 1,1 dioxide |
| CAS Registration Number | [81-07-2] |
| Empirical Formula | C7H5NO3S |
| Molecular weight | 183.18 |
| Regulatory Status | PhEur; USP-NF; JPE |
Physicochemical Properties
| Physical form | Solid, powder |
| Acidity/alkalinity | pH = 2.0 (0.35% w/v aqueous solution) |
| Log P | 0.9 |
| pKa | 1.31 at 25 oC |
| Density (bulk) | 0.7 – 1.0 g/cm3 |
| Melting point oC | 228 – 230 oC (Decomposes) |
| Density (tapped) | 0.9 – 1.2g/cm3 |
| Dissociation constant | pKa = 1.6 at 25 |
| Heat of combustion | 3644.3Kj/mol |
| Moisture content | 0.1% |
| Solubility | 1 in 25 (water); 1 in 50 (glycerin). Slightly soluble in ethanol, acetone and DMF. Soluble in dilute alkali hydroxide solutions |
Applications in Pharmaceutical Formulation
Saccharin is a sweetening agent. It is used as an intense sweetening agent in beverages, food products, table – top sweeteners and oral hygiene products such as toothpastes and mouthwashes.
In oral pharmaceutical formulations, it is used at a concentration of 0.02 – 0.5% w/w. it has been used in chewable tablet formulation as a sweetening agent. Saccharin has been used to form various pharmaceutical co-crystals.
Saccharin can be used to mask some unpleasant taste characteristics or to enhance flavor systems. Its sweetening power is approximately 300 – 600 times that of sucrose.
Pharmacopoeial Specifications
| Test | Specification | Reference |
| Identification | A, B, C, D, E | USP-NF/PhEur |
| Characters | White, crystalline powder or colourless crystals | USP-NF/PhEur |
| Appearance of solution | + | USP-NF/ PhEur |
| o and p-Toluenesulphonamide | + | USP-NF/PhEur |
| Heavy metals | ≤ 10 ppm | USP-NF/PhEur |
| Readily carbonizable substances | + | USP-NF/PhEur |
| Benzoic and salicylic acids | + | USP-NF/PhEur |
| Melting range | ≤ 5ppm | USP-NF/PhEur |
| Loss on drying | ≤1.0% | USP-NF/PhEur |
| Sulphated ash | ≤ 0.2% | |
| Assay (dried basis) | 99.0 – 101.0% | USP-NF/PhEur |
Safety and Regulatory Status
Saccharin is listed in the pharmacopoeia. It is also accepted for use as a food additive in Europe. Note that the E – number “E954” is applied to both saccharin and saccharin salts. Saccharin is also included in the FDA Inactive Ingredients Database (oral solutions, syrups, tablets and topical preparations). A specification for saccharin is contained in the Food Chemicals Codex (FCC).
There has been considerable controversy concerning the safety of saccharin which has led to extensive studies since the mid-1970s.
Two generations studies in rats exposed to diets containing 5.0-7.5% total saccharin (equivalent to 175g daily in humans) suggested that the incidence of bladder tumors was significantly greater in saccharin – treated males of the second generation than in controls. Further experiments in rats suggested that a contaminant of commercial saccharin, o-toluene sulfonamide, might also account for carcinogenic effects. In view of these studies, a ban on the use of saccharin was proposed in several countries. However in 1977 a ban by the FDA led to a congressional moratorium that permitted the continued use of saccharin in the USA.
From the available data is now appears that the development of tumors is a sex, species, and organ – specific phenomenon and extensive epidemiological studies have shown that saccharin intake is not related to bladder cancer in humans.
The WHO has set a temporary acceptable daily intake for saccharin, including its calcium, potassium and sodium salts at up to 2.5mg/kg body weight. In the UK, the Committee On Toxicity of Chemicals in Food, Consumer Products, and the Environment (COT) has set an acceptable daily intake for saccharin and its calcium, potassium and sodium salts (expressed as saccharin sodium) at up to 5mg/kg body weight.
Adverse reactions to saccharin, although relatively few in relation to its widespread use, include: urticarial with pruritus following ingestion of saccharin-sweetened beverages and photosensitization reactions.
LD50 (mouse, oral): 17.5g/kg
LD50 (rat, IP): 7.10g/kg
LD50 (rat, oral): 14.2g/kg
Stability and Storage Conditions
Saccharin is stable under the normal range of conditions employed in formulations. In the bulk form it shows no detectable decomposition and only when it is exposed to a high temperature (125 at a low (pH 2) for over 1 hour does significant decomposition occur. The decomposition product formed is (ammonium–o–sulfo) benzoic acid, which is not sweet. The aqueous stability should be stored in a well closed container in a dry place.
Saccharin can react with large molecules, resulting in a precipitate being formed. It does not undergo mallard browning.
Handling Precautions
Observe normal precautions appropriate to the circumstances and quantity of material handled. Eye protection and a dust mask are recommended.
References
[1] T.A. Biemer, Analysis of saccharin, acesulfame-K and sodium cyclamate by high-performance ion chromatography, Journal of Chromatography A, 463 (1989) 463-468.
[2] A.M. Bertorelli, J.V. Czarnowski-Hill, Review of present and future use of nonnutritive sweeteners, The Diabetes Educator, 16 (1990) 415-420.
[3] A. Mortensen, Sweeteners permitted in the European Union: safety aspects, Scandinavian Journal of Food and Nutrition, 50 (2006) 104-116.
[4] K. Woertz, C. Tissen, P. Kleinebudde, J. Breitkreutz, Taste sensing systems (electronic tongues) for pharmaceutical applications, International Journal of Pharmaceutics, 417 (2011) 256-271.
[5] C. Shortt, Authorised EU health claims for intense sweeteners and sugar replacers, Foods, Nutrients and Food Ingredients with Authorised Eu Health Claims, Elsevier2014, pp. 151-176.
[6] J. Walsh, A. Cram, K. Woertz, J. Breitkreutz, G. Winzenburg, R. Turner, C. Tuleu, I. European Formulation, Playing hide and seek with poorly tasting paediatric medicines: do not forget the excipients, Advanced Drug Delivery Reviews, 73 (2014) 14-33.
[7] P.J.C. Sheskey, Walter G; Cable, Colin G, Handbook of Pharmaceutical Excipients – 8th Edition, Pharmaceutical Development and Technology, 18 (2017) 544-544.
[8] T.U.S.P. Convention, Food Chemicals Codex (11th Edition), (2018).