Methacylic Acid-Methyl Methacrylate Copolymer | Uses, Suppliers, and Specifications
Chemical and Physical Particulars
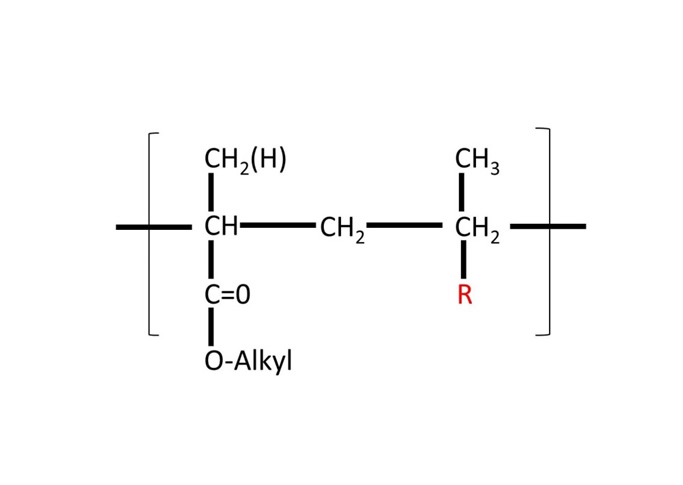
Methacrylic acid – Methyl methacrylate Copolymer (1:2) is synthetic anionic copolymers based on methacrylic acid and methyl methacrylate, having a mean relative molecular mass of approximately 135 000. The ratio of carboxylic groups to ester groups is 1:2. It contains between 27.6% and 30.7% m/m methacrylic acid units. It is supplied as a solid substance in form of a white, free-flowing powder with a faint characteristic odour.
Physicochemical Properties
| Physical state | Solid (powder) |
| Acid value | 180-200 |
| Density (bulk) | 0.390 g/cm3 |
| Density (tapped) | 0.424 g/cm3 |
| Density (true) | 0.83 – 0.85 g/cm3 (Eudragit S) |
| Refractive index | index = 1.39-1..395 |
| Solubility | Acetone, Ethanol & intestinal fluids ³7.0 |
| Viscosity (dynamic) | 50-200 mPa.s |
Applications in Pharmaceutical Formulation
Methacrylic acid – Methyl methacrylate Copolymer (1:2) is an enteric film forming agent and tablet binder. It is used in oral capsule and tablet formulations as film-coating agents. Methacrylic acid – Methyl methacrylate Copolymer (1:2) may additionally be used to form the matrix tablet systems.
The benefits of using Methacrylic acid – Methyl methacrylate Copolymer (1:2) are:
- pH-dependent drug release
- protection of active ingredients that are sensitive to gastric fluids
- protection against mucosal irritation by intolerable formulation
- targeted delivery to the ileum
Pharmacopoeial Specifications
PhEur monograph
| Test | Specification | Reference |
| Identification | A, B | PhEur |
| Characters | White, free-flowing powder | PhEur |
| Appearance of a film | + | PhEur |
| Apparent viscosity | ³50 ≤200 mPa.s | PhEur |
| Methyl acrylate and methacrylic acid | ≤0.1% | PhEur |
| Heavy Metals | – | PhEur |
| Loss on Drying | ≤5.0% | PhEur |
| Sulphated Ash | ≤0.1% | PhEur |
USP-NF monograph
| Test | Specification | Reference |
| Identification | + | USP-NF |
| Viscosity | 50 – 200 mPa.s | USP-NF |
| Loss on drying | ≤5.0% | USP-NF |
| Residue on ignition | ≤0.1% | USP-NF |
| Heavy metals | ≤0.002% | USP-NF |
| Limit of total monomers | ≤0.05% | USP-NF |
| Assay (dried basis) Methacrylic acid units | 27.6 – 30.7% | USP-NF |
Safety and Regulatory Status
Methacrylic acid – Methyl methacrylate Copolymer (1:2) is listed in the PhEur (Methacrylic acid – Methyl methacrylate Copolymer (1:2)), USP-NF (Methacrylic Acid Copoymer, Type B) and the JPE (Methacylic Acid Copolymer S). It is included in the FDA Inactive Ingredients Database (oral capsules and tablets).
Methacrylic acid – Methyl methacrylate Copolymer (1:2) is widely used as film-coating materials in oral pharmaceutical formulations. They are also used in topical formulations and are generally regarded as nontoxic and non-irritant materials.
Based on relevant chronic oral toxicity studies in rats and conventionally calculated with a safety factor of 100, a daily intake of 2-200 mg/kg body-weight depending on the grade.
Stability and Storage Conditions
Dry powder polymer forms are stable at temperatures less than30C. Above this temperature, powders tend to form clumps, although this does not affect the quality of the substance and any clumps formed can he readily broken up. Dry powders arc stable for at least 3 years if stored in a tightly closed container at less than 30C.
Dispersions are sensitive to extreme temperature and phase separation occurs below 0C. Dispersions should therefore be stored at temperatures between 5 and 25C and are stable for at least 18 months after shipping from the manufacturer’s warehouse if stored in a tightly closed container at the above conditions.
LD50 (dog, SC): 4.5g/kg
LD50 (mouse, IP: 1g/kg
LD50(mouse, oral): 5.2 g/kg
LD50(mouse SC.): 6.3g/kg
LD50 (rat. lP(: 1.33 g/kg
LD50(rat, SC): 75g/kg
Handling Precautions
Observe normal precautions appropriate to the circumstances and quantity of material handled. Additional measures should be taken when handling organic solutions of polymethacrylates. Eye protection, gloves, and a dust mask or respirator are recommended.
The materials should be handled in a well-ventilated environment and measures should be taken to prevent dust formation.
Acute and chronic adverse effects have been observed in workers handling the related substances methyl methacrylate and poly(methyl methacrylate) (PMMA). In the UK, the workplace exposure limit for methyl methacrylate has been set at 208 mg/m3 (50ppm) long-term (8-hour TWA), and 416mg/m3 (100ppm) short-term.
References
[1] C.G. Cameron, J.W. McGinity, Controlled-Release Theophylline Tablet Formulations Containing Acrylic Resins, III. Influence of Filler Excipient, Drug Development and Industrial Pharmacy, 13 (1987) 303-318.
[2] M.Z.I. Khan, Ž. Prebeg, N. Kurjaković, A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation Of drug release using Eudragit L100-55 and Eudragit S100 combinations, journal of controlled release, 58 (1999) 215-222.
[3] A. Ceballos, M. Cirri, F. Maestrelli, G. Corti, P. Mura, Influence of formulation and process variables on in vitro release of theophylline from directly-compressed Eudragit matrix tablets, farmaco, 60 (2005) 913-918.
[4] Evonik Industries, EUDRAGIT® L 100, Technical information, (2012).
[5] S. Thakral, N.K. Thakral, D.K. Majumdar, Eudragit®: a technology evaluation, Expert opinion on drug delivery, 10 (2013) 131-149.
[6] R. Wulff, C.S. Leopold, Coatings of Eudragit® RL and L-55 blends: investigations on the drug release mechanism, AAPS PharmSciTech, 17 (2016) 493-503.