| Weight | 5 kg |
|---|
Ethyl Oleate Excipient | Uses, Suppliers, and Specifications
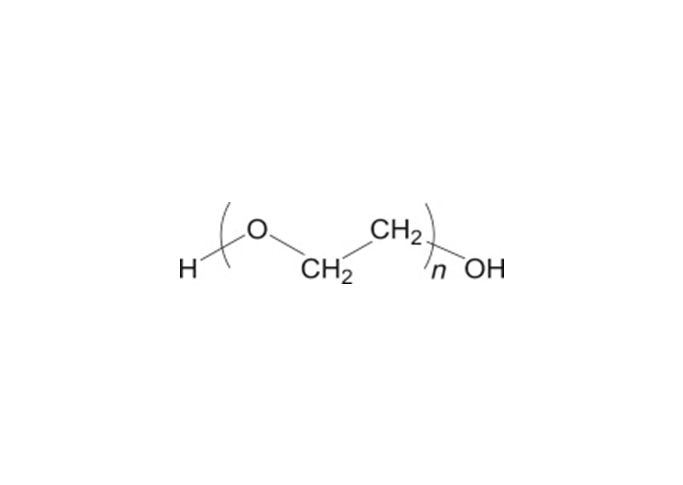
Polyethylene glycol is a polyether polymer compound formed by the reaction of ethylene oxide and water under pressure in the presence of a catalyst. Polyethylene oxide (PEO) and polyethylene glycol have the same CAS registry number 25322-68-3. Both PEO and PEG are non-ionic homopolymers of ethylene oxide, sharing the same formula (CH2CH2O)n where n represents the average number of oxyethylene groups, with n=5–182 for typical PEGs (avg. MW 200-8000) and much larger n’s for PEOs (avg. MW 100,000 to several million). The USP-NF describes polyethylene glycol as an addition polymer of ethylene oxide and water.
Polyethylene glycol grades 200-600 are liquids while grades 1000 and above are solids at ambient temperatures. Liquid grades (PEG 200-600) occur as clear, colourless or slightly yellow-coloured, viscous liquids. They have a slight but characteristic odour and a bitter, slightly burning taste. PEG 600 can occur as a solid at ambient temperatures. Solid grades (PEG>1000) are white or off-white in colour, and range in consistency from pastes to waxy flakes. They have a faint, sweet odour. Grades of PEG 6000 and above are available as free- flowing milled powders.
An alternative empirical formula HOCH2CH2OCH2)mCH2OH can be used where m represents the average number of oxyethylene groups. In the more general formula H(OCH2CHCH2)nOH, n is m+1. The average molecular weights of typical polyethylene glycols is shown in the table below. Note that the number that follows the PEG is an indication of the average molecular weight of the polymer.
| Grade | m | Average molecular weight |
| PEG 200 | 4.2 | 190 – 210 |
| PEG 300 | 6.4 | 285 – 315 |
| PEG 400 | 8.7 | 380 – 420 |
| PEG 600 | 13.2 | 570 – 613 |
| PEG 900 | 15.3 | 855 – 900 |
| PEG 1 000 | 22.3 | 950 – 1050 |
| PEG 2 000 | 40.0 – 50.0 | 1800 – 2200 |
| PEG 3 000 | 60.0 – 75.0 | 2700 – 3300 |
| PEG 3 350 | 75.7 | 3000 – 3700 |
| PEG 6 000 | 136 | 5000 – 7000 |
| PEG 8 000 | 181.4 | 7000 – 9000 |
| PEG 20 000 |
Physicochemical Properties
| Physical form | Liquid to waxy solid |
| Density | 1.11 – 1.144g/cm3 at 25 C for liquid PEGs; 1.15-1.21 g/cm3 at 25 C for solid PEGs. |
| Flash point | 182 oC for PEG 200 to 250 oC for PEG 600 |
| Freezing point | < – 65 oC PEG200 (sets to a glass); -15 to – 8 oC for PEG 300; 4-8 oC for PEG 400; 15-25 oC for PEG 600. |
| Melting point | 37-40 oC for PEG 1000; 44-48 oC for PEG1500; 55-63 oC for PEG 6000; 60-63 oC for PEG 8000; 60-63 oC for PEG 20000. |
| Moisture content
|
Liquid polyethylene glycols are very hygroscopic, although hygroscopicity decreases with increasing molecular weight. Solid grades e.g. PEG 4000 and above, are not hygroscopic |
| Particle size distribution | For solids, |
| Refractive index | 1.459 for PEG 200; = 1.463 for PEG 300; = 1.465 for PEG 400; = 1.467 for PEG 600. |
| Solubility | All grades of polyethylene glycol are soluble in water and miscible in all proportions with other polyethylene glycols (after melting, if necessary). Aqueous solutions of higher- molecular-weight grades may form gels, liquid polyethylene glycols are soluble in alcohols, glycerin and glycols. Solid polyethylene glycols are soluble in acetone, ethanol (95%), and methanol; they are insoluble in fats, fixed oils and mineral oil. |
| Surface tension | Approximately 44 mN/m for liquid polyethylene glvcols; approximately 55 mN/m for 10% w/v aqueous solution of solid polyethylene glycol. |
| Viscosity (kinematic) | 3.9 – 4.8 mm2/s for PEG 200; 5.4 – 6.4 mm2/s for PEG 300; 6.8 – 8.0 mm2/s for PEG 400. See monograph for other grades. |
Applications in Pharmaceutical Formulations
Polyethylene glycols are ointment bases, plasticizers, solvents; suppository bases and tablet and capsule lubricants.
Polyethylene glycols are widely used in a variety of pharmaceutical formulations, including parenteral, topicaI, ophthalmic, oral, and rectal preparations.
Polyethylene glycols are stable, hydrophilic substances that are essentially non-irritant to the skin. They do not readily penetrate the skin, although the polyethylene glycols are water-soluble and are easily removed from the skin by washing, making them useful as ointment bases. Solid grades are generally employed in topical ointments, with the consistency of the base being modified by the addition of liquid grades of polyethylene glycol.
Mixtures of polyethylene glycols can be used as suppository bases, for which they have many advantages over fats. For example, the melting point of the suppository can be made higher to withstand exposure to warmer climates: release of the drug is not dependent upon melting point, the physical stability on storage is better; and suppositories are readily miscible with rectal fluids.
Aqueous polyethylene glycol solutions can he used either as suspending agents or to adjust the viscosity and consistency of other suspending vehicles. When used in conjunction with other emulsifiers, polyethylene glycols can act as emulsion stabilizers.
Liquid polyethylene glycois are used as water-miscible solvents for the contents of soft gelatin capsules. However they may cause hardening of the capsule shells by preferential absorption of moisture from gelatin in the shell.
In solid-dosage formulations, higher-molecular-weight polyethylene glycols can enhance the effectiveness of tablet binders and impart plasticity to granules. However, they have only limited binding action when used alone, and can prolong disintegration if present in concentrations greater than 5% w/w.
When used for thermoplastic a mixture of the powdered constituents with 10-15% w/w PEG 6000 is heated to 70-55 C. The mass becomes pastelike and forms granules if stirred while cooling. This technique is useful for the preparation of dosage forms such as lozenges when prolonged disintegration is required.
Polyethylene glycols can also be used to enhance the aqueous solubility or dissolution characteristics of poorly soluble compounds by making solid dispersions with an appropriate polyethylene glycol.
In film coatings, solid grades of polyethylene glycol can be used alone for the film-coating of tablets or can be useful as hydrophilic polishing materials. Solid grades are also widely used as plasticizers in conjunction with film-forming polymers. The presence of polyethylene glycol in film coats, especially of liquid grades, tends to increase their water permeability and may reduce protection against low pH in enteric-coating films. Polyethylene glycols are useful as plasticizers in microencapsulated products to avoid rupture of the coating film when the microcapsules are compressed into tablets.
Polyethylene glycol grades with molecular weights of 6000 and above can be used as lubricants, particularly for soluble tablets. The lubricant action is not as good as that of magnesium stearate, and stickiness may develop if the material becomes too warm during compression. An anti-adherent effect is also exerted, again subject to the avoidance of overheating.
Pharmacopoeial Specifications
| Test | Specification | Reference |
| Identification | A, B, C | USP-NF/PhEur |
| Appearance | Clear, viscous, colourless or almost colourless hygroscopic liquids; white or almost white hygroscopic solids with a waxy appearance | USP-NF/PhEur |
| Acidity or alkalinity | ≤0.1 M NaOH | USP-NF/PhEur |
| Dynamic viscosity (mPa.s)
300 400 600 1 000 1 500 3 000 3 350 6 000 20 000 |
80 – 105 105 – 130 15 – 20 22 – 30 34 – 50 75 – 100 83 – 120 200 – 270 2700 – 3500 |
USP-NF/PhEur |
| Density (g/ml)
300 400 600 1 000 1 500 3 000 3 350 6 000 2 0000 |
1.120 1.120 1.080 1.080 1.080 1.080 1.080 1.080 1.080 |
USP-NF/ PhEur |
| Hydroxyl value
300 400 600 1 000 1 500 3 000 3 350 6 000 20 000 |
340 – 394 264 – 300 178 – 197 107 – 118 70 – 80 34 – 42 30 – 38 16 – 22 – |
USP-NF/PhEur |
| Reducing substances | + | USP-NF/PhEur |
| Formaldehyde | ≤15ppm | USP-NF/PhEur |
| Ethylene glycol and diethylene glycol | ≤0.4% | USP-NF/PhEur |
| Ethylene oxide and dioxan | ≤10ppm | |
| Heavy metals | ≤20ppm | USP-NF/PhEur |
| Water | ≤2% | USP-NF/PhEur |
| Sulphated ash | ≤0.2% | USP-NF/PhEur |
Safely and Regulatory Status
Polyethylene glycols are listed in all major pharmacopoeia. They are also included in the FDA Inactive Ingredients Database (dental preparations; IM and IV infections; ophthalmic preparations; oral capsules, solutions. syrups, and tablets; rectal, tipical, anid vaginal preparations).
Polyethylene glycols are widely used in a variety of pharmaceutical formulations. Generally, they are regarded as nontoxic and non-irritant materials.
Adverse reactions to polyethylene are relatively low. However, polyethylene glycols administered topically may cause stinging, especially when applied to mucous membranes. Hypersensitivity reactions to polyethylene glycols applied topically have also been reported, including urticaria and delayed allergic reacrions.
The most serious adverse effects associated with polyethylene glycols are hyperosmolarity, metabolic acidosis, and renal failure following the topical use of polyethylene glycols in burn patients. Topical preparations containing polyethylene glycols should therefore be used cautiously in patients with renal failure, extensive burns, or open wounds.
Oral administration of large quantities of polyethylene glycols can have a laxative effect Therapeutically, up to 4L of an aqueous mixture of electrolytes and high-molecular-weight polyethylene glycol is consumed by patients undergoing bowel cleansing.
Liquid polyethylene glycols may be absorbed when taken orally but the higher-molecular-weight polyethylene glycols are not significantly absorbed from the gastrointestinal tract. Absorbed polyethylene glycol is excreted largely unchanged in the urine, although polyethylene glycol of low molecular weight may be partially metabolized.
The WHO has set an estimated acceptable daily intake of polyethylene glycols at up to 10mg/kg body-weight.
In parenteral products, the maximum recommended concentration of PEG 300 is approximately 30% v/v as hemolytic effects has been observed at concentrations greater than about 40% v/v.
Stability and Storage Conditions
Polyethylene glycols are chemically stable in air and in solution, although grades with a molecular weight less than 2000 are hygroscopic. Polyethylene glycols do not support microbial growth and the do not become rancid.
Polyethylene glycols and aqueous polyethylene glycol solutions can be sterilized by autoclaving, filtration, or gamma irradiation.
Sterilization of solid grades by dry heat at 150 oC for 1 hour may induce oxidation, darkening, and the formation of acidic degradation products. Ideally, sterilization should be carried out in an inert atmosphere. Oxidation of polyethylene glycol may also be inhibited by the inclusion of a suitable antioxidant.
If heated tanks arc used to maintain normally solid polyethylene glycols in a molten state, care must be taken to avoid contamination with iron, which can lead to discoloration. The temperature must be kept to the minimum necessary to ensure fluidity; oxidation may occur if polyethylene glycols are exposed for long periods to temperatures exceeding 50 C. However, storage under nitrogen reduces the possibility of oxidation.
Polyethylene glycol should he stored in well-closed containers in a cool, dry place. Stainless steel, aluminium, glass, or lined steel containers are preferred for the storage of liquid grades.
The chemical reactivity of polyethylene glycols is mainly confined to the two terminal hydroxyl groups, which can be either esterified. However, all grades can exhibit some oxidizing activity owing to the presence of peroxide impurities and secondary products formed by autoxidation,
Liquid and solid polyethylene glycol grades may be incompatible with some colouring agents. The antibacterial activity of certain antibiotics is reduced in polyethylene glycol bases, particularly that of penicillin and bacitracin. The preservative efficacy of the parabens may also be impaired owing to binding with polyethylene glycols.
Physical effects caused by polyethylene glycol bases include softening and liquefaction in mixtures with salicylic acid. Discoloration of sulphonamides can also occur, and sorbitol may be precipitated from mixtures. Plastics, such as polyethylene, polyvinyl chloride, and cellulose-ester membranes (in filters) may be softened or dissolved in polyethylene glycols. Migration of polyethylene glycol can occur from tablet film coatings, leading to interaction with core components
Handling Precautions
Observe normal precautions appropriate to the circumstances and quantity of material handled. Eye protection is recommended.
References
[1] R. Kaur, D.J.W. Grant, T. Eaves, Comparison of polyethylene glycol and polyoxyethylene stearate as excipients for solid dispersion systems of griseofulvin and tolbutamide I: Phase equilibria, Journal of Pharmaceutical Sciences, 69 (1980) 1317-1321.
[2] M.R. Jafari, A.G. Danti, I. Ahmed, Comparison of polyethylene glycol, polyvinylpyrrolidone and urea as excipients for solid dispersion systems of miconazole nitrate, International Journal of Pharmaceutics, 48 (1988) 207-215.
[3] A.J. Peacock, Handbook of Polyethylene: Structures: Properties, and Applications, 2000.
[4] R. N, R. E, Effect of drug solubility on in vitro availability rate from suppositories with polyethylene glycol excipients, Die Pharmazie, 56 (2001) 163-167.
[5] R.G. Strickley, Solubilizing Excipients in Oral and Injectable Formulations, Pharmaceutical Research, 21 (2004) 201-230.
[6] W.R. Wasylaschuk, P.A. Harmon, G. Wagner, A.B. Harman, A.C. Templeton, H. Xu, R.A. Reed, Evaluation of Hydroperoxides in Common Pharmaceutical Excipients, Journal of Pharmaceutical Sciences, 96 (2007) 106-116.