Opinion | What Does the Recent Pig-to-Human Kidney Transplant Mean for Tissue Therapeutics?
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...
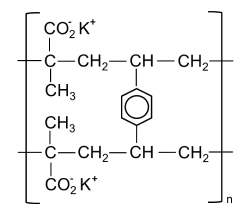
Polacrilin Potassium is the potassium salt of 2-methyl-2-propenoic acid polymer with divinylbenzene methacrylic acid. It is a cation-exchange resin approved for use in oral pharmaceutical products as an excipient. Polacrilin Potassium is available as a cream-coloured, odourless, and taste-less free-flowing powder.
Pharmacopoeial Compliance: USP-NF
Synonyms and Trade Names: Polacrilin Potassium; Methacrylic Acid Polymer with Divinylbenzene Potassium Salt; 2-Methyl-2-Propenoic Acid Polymer with Divinylbenzene Potassium Salt; Amberlite® IR P-88; KYRON® 314; PUROLITE® C115 KMR
Uses and Applications: Tablet and Capsule Disintegrant
Polacrilin potassium is the potassium salt of a unifunctional low-cross-linked carboxylic cation-exchange resin prepared from methacrylic acid and divinylbenzene. Polacrilin resin is prepared by the copolymerization of methacrylic acid with divinylbenzcine (DVB). Polacrilin potassium is then produced by neutralizing this resin with potassium hydroxide. When previously dried at 105 °Cfor 6 hours, the substance obtained contains not less than 20.6 per cent and not more than 25.1 per cent of potassium.
Materials generally classified as ion exchange resins have a long history of use. They were developed by Rohm and Haas (now a subsidiary of Dow Chemical) based on methacrylic acid chemistry between the 1930s and 1950s. Polacrilin potassium, which was among the first of the ion exchange resins adapted for use in the pharmaceutical industry, is weakly acidic, absorbs water rapidly in aqueous media owing to its hydrophilic nature, and swells significantly in the process.
Since the swelling capacity is significant, when incorporated in tablet formulations, Polacrilin potassium generates a strong force capable of disrupting and disintegrating tablets even when compressed at very high compaction pressures. It is for this reason that Haas and Rohm commercialised Polacrilin potassium (under the brand name Amberlite® IRP88) as a disintegrating agent.
Polacrilin potassium is supplied as a cream-coloured, virtually odourless and tasteless, free-flowing powder that may be smooth or gritty depending on the particle size and material grade.
| Chemical Name | Potassium 1,2-bis(ethenyl)benzene 2-methylprop-2-enoate |
| CAS Registration Number | [39394-76-5] and [65405-55-2] |
| Empirical Formula | C14H15KO2 |
| Molecular weight | 254.37 g/mol (monomer) |
| EINCES Number | 809-624-5 |
| UNII Code (FDA) | 0BZ5A00FQU |
Polacrilin potassium is an approved pharmaceutical excipient. it is currently listed in the USP-NF and also included in the FDA Inactive Ingredients Database (for oral capsules and tablets). Polacrilin potassium (and related Polacrilin resins) are widely used in oral pharmaceutical formulations and are generally regarded as nontoxic and non-irritant materials.
| Physical form | Solid, powder |
| Bulk density | 0.48 g/cm for Amberlite IRP-88 |
| Tapped density | 0.62 g/cnm3 for Amberlite IRP-88 |
| Particle size distribution | 0.075 – 0.150 mm (30% maximum); >0.150 mm (1.0% maximum) |
| pH range | 5 – 4 |
| Moisture content | ≈10% |
| Solubility | Practically insoluble in water and most other solvents. Swell significantly when exposed to aqueous environments |
| Specification | Reference | |
| Name | Polacrilin Potassium | |
| Authorised use | Excipient | |
| Identification | A
B |
USP-NF |
| Characters | specified | |
| Loss on drying | ≤ 10.0% | USP-NF |
| Powder fineness | ≤ 1.0% on a #100 mesh; ≤ 30.0% on a #200 mesh | USP-NF |
| Iron | ≤ 0.01% | USP-NF |
| Sodium | ≤ 0.20% | USP-NF |
| Heavy Metals | ≤ 2 ppm | USP-NF |
| Assay for potassium | 20.6% – 25.1% | USP-NF |
| Labelling | n/a | USP-NF |
Polacrilin potassium is a cation-exchange resin mainly used in oral solid dosage forms as a disintegrant. It is added at concentrations of 2-10% w/w, although 2% w/w is usually adequate. Note that Polacrilin potassium is most effective in hydrophobic tablet formulations in which conventional disintegrating agents are less effective. It has also been used to formulate Orally Disintegrating Tablets.
There are other Polacrilin ion-exchange resins are commercially available for use as excipients (for instance, to stabilize drug substances, mask or modify bitter tasting drugs, and formulate sustained-release dosage forms). A number of these have separate monographs and are therefore distinct from Polacrilin potassium which is discussed in this monograph.
It has been reported that Polacrilin resins may also be used in the preparation and/or analysis of pharmaceuticals and food products (for instance, to extract dyes from food substances, and isolate and identify trace substances in products).
Finally, several other ionic exchange resins are commercially available and are used in various pharmaceutical applications. They are mainly differentiated by the acidity or basicity of the functional groups, which results in strongly acidic to strongly basic ion-exchange resin types. This is illustrated in the table below for Amberlite® ion-exchange resins from Dupont:
| Cation-exchange resins | Anion-exchange resins | ||||
| Amberlite Grade | IRP-69 | IRP-64 | IRP-88 | IRP-58 | IRP-67 |
| Copolymer | Styrene and DVB | Methacrylic acid and DVB | Methacrylic acid and DVB | Phenolic polyamine | Styrene and DVB |
| Type | Strongly acidic | Weakly acidic | Weakly acidic | Weakly basic | Strongly basic |
| Functional structure | SO3 Na+ | COO–H+ | COO– K+ | NH2NH2 | NCH3)3+ Cl- Cl- |
| Ionic form | Na+ | H+ | K+ | Free base | Cl– |
| Particle size (µm) | 100 – 500 | 100 – 500 | 100 – 500 | 100 – 500 | 100 – 500 |
| Parent resin | IR-120 | IRC-50 | IRC-50 | IR-4B | IRA-400 |
| pH range | 0 – 14 | 5 – 14 | 5 – 14 | 0 – 7 | 0 – 12 |
| Application | Carrier for cationic drugs that are bases or salts | Carrier for cationic drugs that are bases or salts | Tablet disintegrant | Carrier for anionic drugs that are acids or salts | Carrier for anionic drugs that are acids or salts |
Polacrilin potassium and other ion-exchange resins are approved for use in oral pharmaceutical formulations and are generally regarded as nontoxic and non-irritant materials. However, ingestion of Polacrilin resins in large amounts can disturb the body’s electrolyte balance, and should therefore be avoided.
Under standard conditions, Polacrilin potassium remains stable and is not significantly affected by exposure to light, air, and heat (up to its maximum toleration temperature). Exposure to excess heat stress can cause thermal decomposition of the material. For this reason, Polacrilin potassium should be stored in tightly closed containers and away from moisture or heat. When handling Polacrilin potassium workers are advised to observe applicable SHEQ protocols appropriate to the amount of material being processed. As Polacrilin potassium may be irritating to the eyes and mucous membranes, the use of eye protection and gloves is advised.
Polacrilin Potassium has not been assessed by the Excipients Forum and assigned a score.
In a pioneering procedure, a team of surgeons at New York University Langone Health Grossman School of Medicine in New...

[bsa_pro_ad_space id=2]
[bsa_pro_ad_space id=4]
PharmaCentral.com may on occasion publish user-generated content. Any information provided on our platform is for general informational and educational purposes only. All information is provided in good faith to enable collaboration and sharing of know-how among our community of users. Authors who submit content retain copyright to it.
PharmaCentral.com does not make any representation or warranty of any kind regarding its accuracy, adequacy, or legality. Any references to particular product names, brands, descriptions, formats, styles, corporate entities, tests, applications, technologies, uses, standardisations, medical conditions, and treatments are for illustration purposes and should not be considered complete or binding. All respective intellectual property, such as trademarks and logos, are properties of their owners
Under Section 107 of the Copyright Act 1976, allowance is made for ‘Fair Use’ for purposes such as criticism, comment, news, reporting, scholarship, education, and research.
Fair use is a use permitted by copyright statute that might otherwise be infringing.
Some information contained on PharmaCentral.com may contain copyrighted material, the use of which may not have been specifically authorised by the respective copyright owners. Some material is made available to help explain and relay complex phenomena, formulae, physical and chemical constants, and other concepts that are scientifically incontestable but relevant to the use of products, and/or to illustrate, transmit, and teach pharmaceutical science principles. Some material is published to support research and user education, and for the public good.