Avicel® is a brand name by Dupont® for grades of Microcrystalline cellulose. Avicel® is one of the best-known pharmaceutical excipient brands and, without doubt, one of the most frequently used raw materials in solid oral product formulation and manufacture. However, with many different grades (standard and specialty) on offer, how do you select the correct material for your needs? To help, we’ve put together a simple guide for selecting Avicel® Microcrystalline cellulose grades, with simple explanations of some of the most common grades, plus recommendations that will serve the majority of buyers.
What is Avicel® Microcrystalline Cellulose?
Avicel® Microcrystalline cellulose is a type of cellulose, a naturally-occurring, large molecular weight polysaccharide found in woody plants. It is widely used within the pharmaceutical, food, and cosmetic sectors as a formulation aid. Avicel® Microcrystalline cellulose was discovered in 1955 by a team at FMC Corporation, led by the legendary Canadian-born chemist, Orlando Aloysius Battista, which he initially named ‘mechanically disintegrated D.P cellulose. Interestingly, the discovery of Avicel® was accidental, the team was attempting to break up hydrolysed cellulose using a Waring Blender. Instead, they obtained a stable colloidal suspension.
The product would later go on to be commercialized under the brand name Avicel® by FMC Corporation. In the late 1960s, Avicel® Microcrystalline cellulose was registered in the supplement to the National Formulary as a pharmaceutical excipient. More than 65 years later, Avicel® continues to command global acceptance, with no signs of its popularity waning.
The table below lists the most important chemical identifiers applicable to Avicel® Microcrystalline cellulose.
Avicel® Microcrystalline Cellulose: Chemical Identifiers
| Chemical Name: | 4-O-[(1S)-hexopyranosyl]-D-glycero-hexopyranose |
| CAS Registry Number: | [9004-34-61] |
| Empirical Formula: | (C6H10O5)n |
| Molecular Weight: | Approx. 36 000
where n = approx. 220 |
| EINECS Number: | 232-674-9 |
| UNII Code (FDA): | OP1R32D61U |
Cellulose: The backbone of Avicel® Microcrystalline cellulose
Cellulose is a linear polymer of β-D-glucose, linked through carbon atoms 1 and 4. The main source of cellulose is woody plants, since it is the main building block of the cell walls of higher plants. Non-woody sources are also viable sources of cellulose, although only woody plants and cotton are the main sources for microcrystalline cellulose used as a pharmaceutical excipient. The chemical structure of cellulose is shown below:
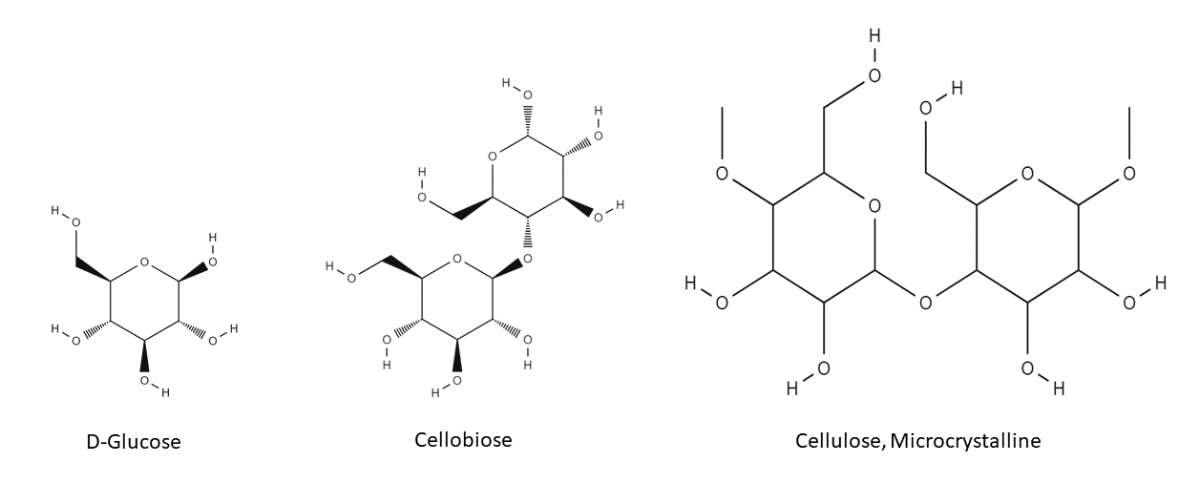
In the biosynthesis of cellulose, two molecules of β-D-glucose molecules combine via a condensation reaction (elimination of one molecule of water) to form the so-called cellobiose unit. The cellulose molecule is formed through repeated condensation of cellobiose units. The formed cellulose molecules pack themselves closely to each other, stabilised through hydrogen bonds, creating crystalline regions interspersed with amorphous regions.
The figure below illustrates the structural organisation of cellulose within plants. The smallest units, the elementary fibrils (cellulose crystalline strands) are made up of many β-(1-4) glucose chains which are bundled to form microfibrils. These, in turn, are assembled into fibrils in the cell wall.
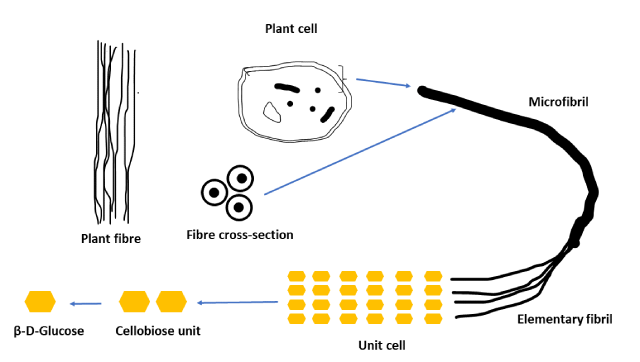
Since cellulose contains both amorphous and crystalline regions, when the crystalline regions are mechanically and chemically isolated and elaborated, various useful ingredients in the form of cellulose crystals are obtained, one of which is Avicel® Microcrystalline cellulose.
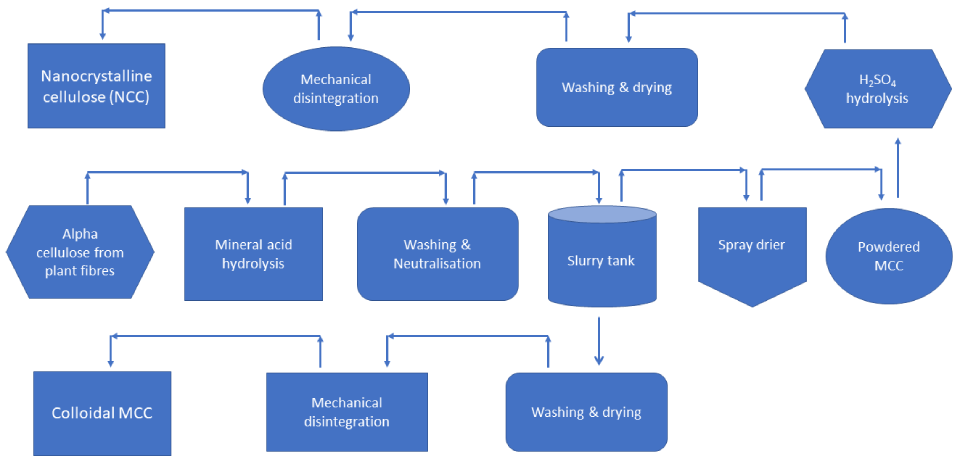
A schematic illustration of the manufacturing process for Avicel® Microcrystalline cellulose is shown below. The process is relatively basic; and starts with treating cellulose, obtained as a high-grade pulp obtained from fibrous plant material, with mineral acids, followed by purification and spray-drying. Further elaborations can then be performed on the obtained material to produce many other grades of Microcrystalline cellulose.
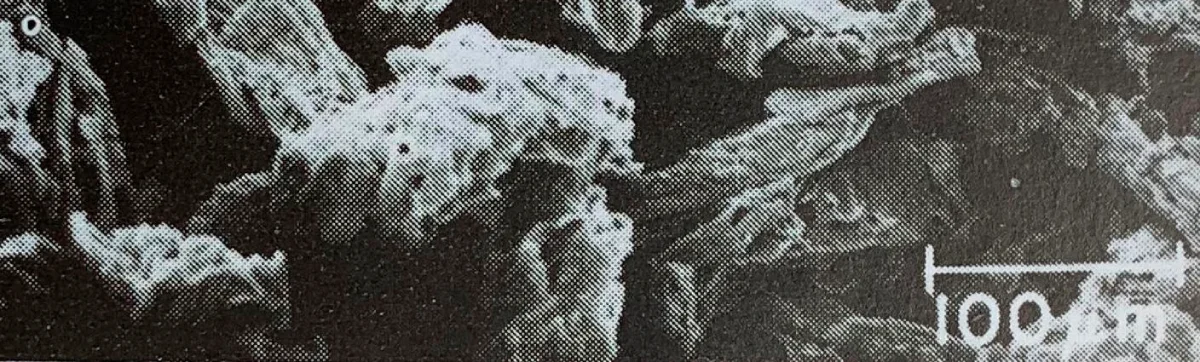
Appearance and Physical Properties of Avicel® Microcrystalline cellulose
Avicel® Microcrystalline cellulose occurs as a white, odourless, tasteless, fibrous powder composed of porous particles. It is hygroscopic and insoluble in water. The table below shows the most common physicochemical parameters of this raw material. Note that Avicel® Microcrystalline cellulose is available in different particle sizes and moisture grades that have different properties and applications. The data shown below apply only to Avicel® PH 101.
Avicel® Microcrystalline Cellulose Physical Properties
| Physical form | Solid, crystalline powder |
| Angle of repose | 30 – 50o depending on grade |
| Density (bulk) | 0.28 – 0.36 g/cm3 |
| Flowability | 1.4 g/s (Avicel® PH-102) |
| Melting point (Chars at) | 260-2700C |
| Moisture content | Hygroscopic. Typically >5% |
| Particle size distribution | The typical mean particle size is 20—200 µm. |
| Solubility | Slightly soluble in 5% w/v sodium hydroxide solution; practically insoluble in water, dilute acids, and most organic solvents. |
| Specific surface area | 0.78 – 1.4 m2/g |
As some of the data above show, microcrystalline cellulose possesses unique physicochemical properties that set it apart from native cellulose. These include amphiphilicity, a higher degree of crystallinity, surface area, higher hydration properties, and morphology. It is also inert and biocompatible. It flows and compresses extremely well, properties that endear this material wide scope of use in many fields.
Avicel® Microcrystalline cellulose Grades
Avicel® Microcrystalline cellulose is available in many grades, which differ in particle size, bulk density, and moisture content. Generally, these can be classified into two broad groups: Standard grades, and Co-processed grades.
Standard Grades
These grades have particle sizes ranging from <30µ to approximately 200. Their nomenclature and recommended applications are shown below:
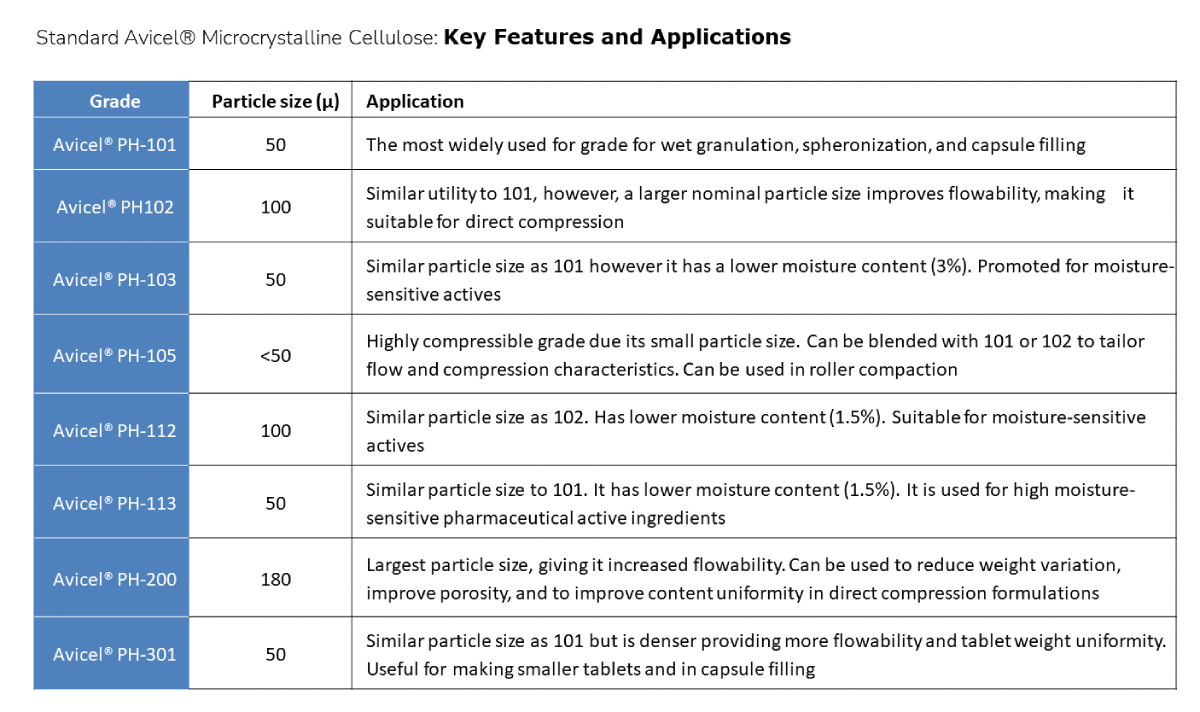
Co-processed Grades
Co-processed Avicel® grades are high functionality materials designed to bring added performance over and above what standard grades offer. The current product range and recommended usage are summarised below:
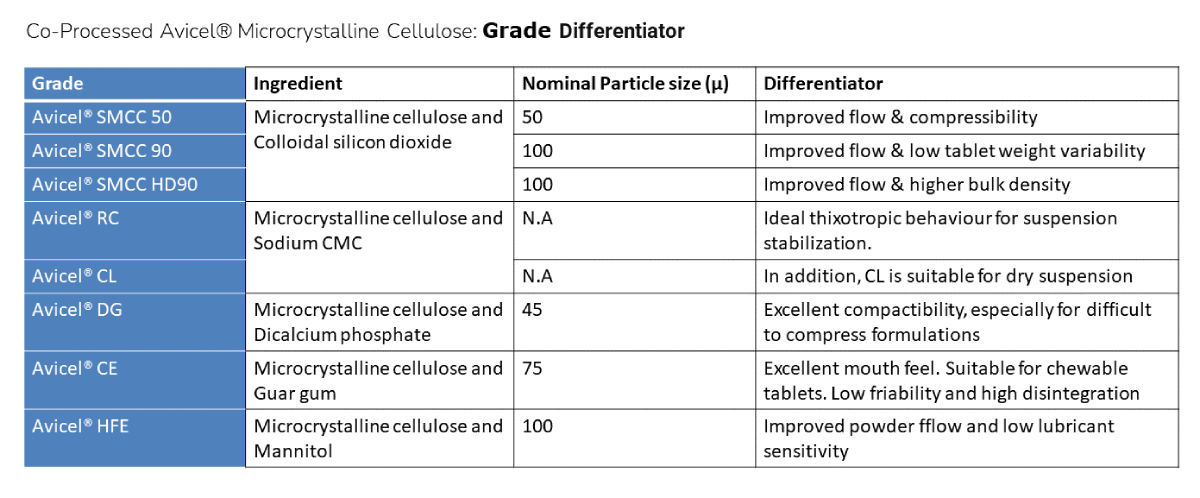
Regulatory Status of Avicel® Microcrystalline Cellulose
Microcrystalline Cellulose is currently listed in the United States Pharmacopoeia and National Formulary, the European Pharmacopeia, the Japanese Pharmacopoeia, the British Pharmacopoeia, in addition to the Indian and Chinese Pharmacopeia, to name but a few. It is an excipient approved for use in pharmaceutical products and is also GRAS listed and accepted for use as a food additive in Europe and the United States and is included in the FDA Inactive Ingredients Database (oral: powders, suspensions, syrups, and tablets; topical & vaginal products).
Uses of Avicel® Microcrystalline Cellulose Grades in Pharmaceutical Products
The application areas for Avicel® Microcrystalline cellulose are immense and encompass many industrial sectors such as food, pharmaceuticals, and cosmetics. Within the pharmaceuticals field, where the bulk of this material is utilised, Avicel® Microcrystalline cellulose functions as a filler and diluent, dry binder, flow, disintegrating, and compression aid in tablets, capsules, and granules. It can be processed both via wet granulation and direct compression, as well as roller compression and extrusion-spheronisation.
Avicel® Microcrystalline cellulose also serves as a moisture adsorbent and as a general-purpose formulation improver. Formulators select it when they need to enhance a formulation’s robustness especially when less compactable diluents and binders are used.
When selected in wet granulation, the optimum level of use is between 5 and 20%, although higher levels have been successfully used. In roller compaction, Avicel® Microcrystalline cellulose improves compaction during the ribbon phase, enabling a trouble-free process while also preserving the plasticity of the compacts. In extrusion-spheronisation, it is the excipient of choice. It works as a moisture sponge and permits the controlled growth of spheroids.
The charts below provide guidance on how to select and use standard and co-processed grades of Avicel® Microcrystalline cellulose in pharmaceutical products.
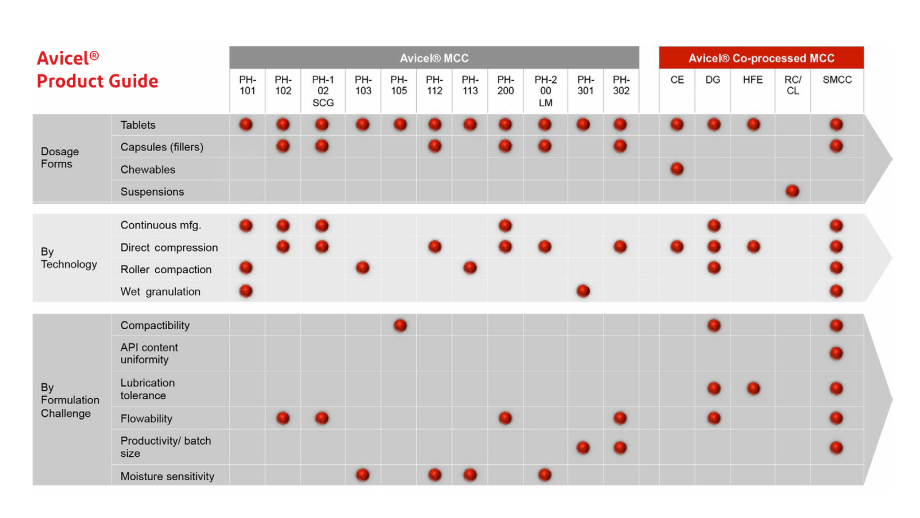
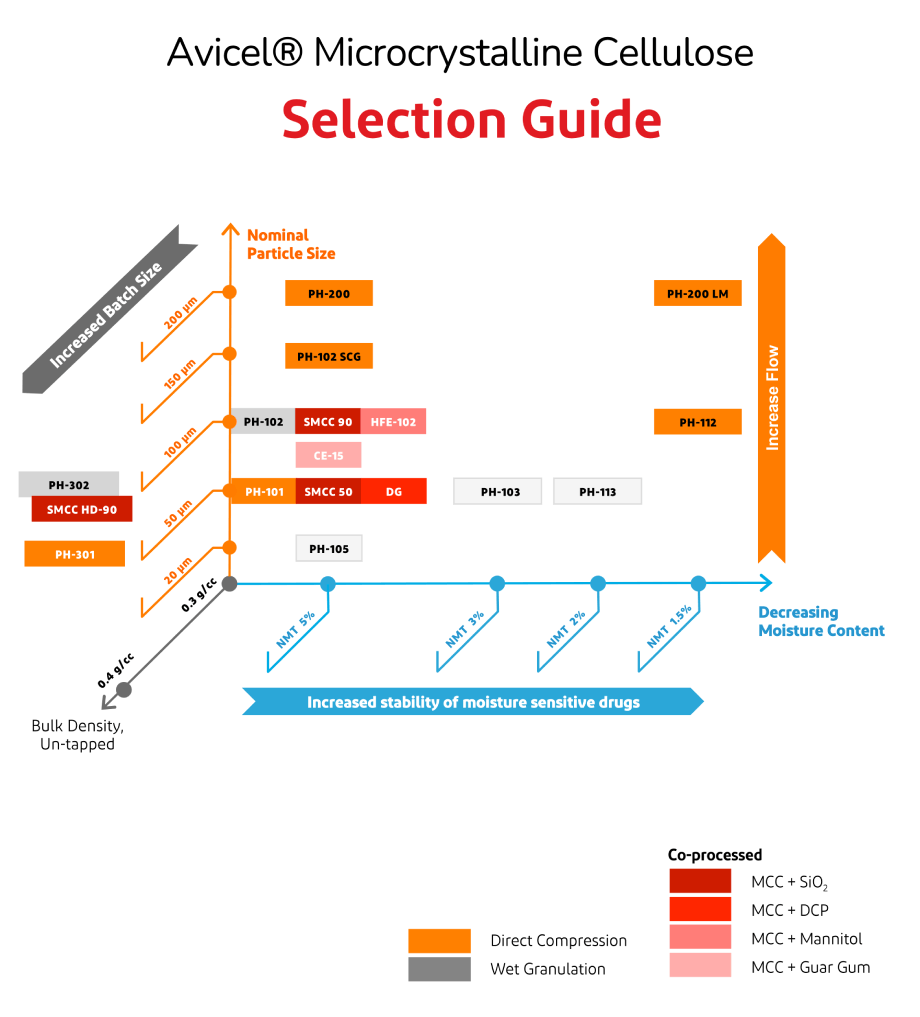
The representative usage of Avicel® PH-101 and Avicel® PH-102 Microcrystalline cellulose in wet granulation and direct compression is shown in the following two examples.
FAQs about Avicel® Microcrystalline cellulose
Is Avicel® Microcrystalline cellulose Safe?
Avicel® Microcrystalline Cellulose is a safe and non-hazardous material that is widely used in pharmaceutical formulations and food products (as a bulking agent and to add crispness). It is generally regarded as a relatively nontoxic and non-irritant material that is safe for human and animal consumption.
Studies from the World Health Organisation and the European Food Safety Agency have concluded that Microcrystalline cellulose has no safety concerns for the public when used in animal nutrition. The United States Food and Drug Administration considers microcrystalline cellulose a safe material for all animal species. Similarly, the European Food Safety Agency has also evaluated Microcrystalline cellulose and established that it is not absorbed systemically and excreted unchanged.
Upon ingestion, microcrystalline cellulose is not absorbed into the blood stream and therefore has little toxicological potential. However, consumption of large quantities of the material may have a laxative effect. This is unlikely to be a problem when Microcrystalline cellulose is consumed as part of a medical product where it is used as an excipient in the formulation.
However, if handling bulk powders, observance of appropriate safeguards is recommended. Gloves, eye protection and a dust mask should be used. The UK Health & Safety Executive has set workplace exposure limits at 10 mg/m3 long-term (8 hr TWA) for total inhaled dust and 4 mg/m3 for respirable dust. The short-term limit for total inhalable dust is 20 mg/m3.
What are the Nutritional Properties of Avicel® Microcrystalline cellulose?
Avicel® Microcrystalline cellulose is composed of insoluble fibre and does not contribute any other material of nutritional importance. Thus, Avicel® contributes no or very few calories to the food system. It is, however, a good source of fibre.
It should be noted that native cellulose itself is completely indigestible, and its hydrolysis during the production of microcrystalline cellulose does not alter its behaviour in the intestine. For this reason, nutritionists regard standard powdered microcrystalline cellulose as having zero energy content.
Although the breakup of cellulose to form colloidal microcrystalline cellulose has no impact on calorific content of the material, colloidal microcrystalline cellulose contain soluble components (hydrocolloids) that are introduced during processing, which add calories to the product.
The table below lists the typical nutritional information of microcrystalline cellulose:
Quantitative nutritional analysis of Avicel® Microcrystalline cellulose (in 100g)1
| Avicel® MCC (powdered) | Colloidal MCC | |
| Energy Content (kcal) | 0 | 20 |
| Fat | 0 | 0 |
| Dietary fibre (total) | 98 | 93 |
| Soluble fibre | 0 | 5 g |
| Carbohydrates (sugars) | N.d (not detected) | N.d (not detected) |
| Protein | N.d (not detected) | N.d (not detected) |
| Vitamin A | N.d (not detected) | N.d (not detected) |
| Vitamin C | N.d (not detected) | N.d (not detected) |
| Sodium | 4 mg | 934 mg |
| Iron | 0.24 mg | 0.5 mg |
| Calcium | 0.1 mg | 2.0 mg |
| Ash | 1 g | 2 g |
In summary, Avicel® microcrystalline cellulose is virtually inert, its energy content is very low or zero and it is not absorbed. However, it yields non-digestible fibre. It does not interfere with other nutrients when added to food products, and is instead, an excellent carrier and stabiliser, helping prolong the viability of food products.
Where to obtain Avicel® Microcrystalline Cellulose
Avicel® Microcrystalline cellulose is manufactured in the United States of America by Dupont. Dupont acquired the microcrystalline cellulose business of FMC Corporation in 2017. Currently, it is marketed both directly and indirectly (through distributors) depending on the country in question. if interested in purchasing or obtaining a sample for assessment, you should contact Dupont themselves directly.
Key References and Citations on Avicel® Microcrystalline cellulose
- F. Lerk, G.K. Bolhuis, A.H. De Boer, Effect of Microcrystalline Cellulose on liquid penetration in and disintegration of directly compressed tablets, Journal of Pharmaceutical Sciences, 68 (1979) 205-211. https://doi.org/10.1002/jps.2600680222. Pubmed | Google Scholar
- Patel, F. Podczeck, Investigation of the effect of type and source of Microcrystalline Cellulose on capsule filling, International Journal of Pharmaceutics, 128 (1996) 123-127. https://doi.org/10.1016/0378-5173(95)04231-8. Pubmed | Google Scholar
- F. Steele, M. Tobyn, S. Edge, A. Chen & J. N. Staniforth.Physicochemical and Mechanical Evaluation of a Novel High Density Grade of Silicified Microcrystalline Cellulose, Drug Development and Industrial Pharmacy, 30:1 (2004), 103-109. https://doi.org/10.1081/DDC-120027517. Pubmed | Google Scholar
- Kristensen, T. Schæfer, P. Kleinebudde, Direct pelletization in a rotary processor controlled by torque measurements. II: effects of changes in the content of Microcrystalline Cellulose, AAPS PharmSci, 2 (2000) 45. https://doi.org/10.1208/ps020324. Pubmed | Google Scholar
- Van Veen, G.K. Bolhuis, Y.S. Wu, K. Zuurman, H.W. Frijlink, Compaction mechanism and tablet strength of unlubricated and lubricated (silicified) Microcrystalline Cellulose, European journal of Pharmaceutics and Biopharmaceutics, 59 (2005) 133-138. https://doi.org/10.1016/j.ejpb.2004.05.009. Pubmed | Google Scholar
- Battista, O. A., and Smith, P. A. (1962). “Microcrystalline cellulose -The oldest polymer finds new industrial uses,” Eng. Chem.54(9), 20-29.
- Microcrystalline Cellulose. In Cellulose and Cellulose Derivatives in the Food Industry, T. Wüstenberg (Ed.). https://doi.org/10.1002/9783527682935.ch04
